Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0P9RF
|
|||
| Former ID |
DAP000371
|
|||
| Drug Name |
Terazosin
|
|||
| Synonyms |
Blavin; Flumarc; Fosfomic; Hytracin; Hytrin; Terazosabb; Terazosina; Terazosine; Terazosinum; Vasomet; Trazosin HCl; A 45975; Abbott 45975; A-45975; Hytrin (TN); Terazosabb (TN); Terazosin (INN); Terazosin [INN:BAN]; Terazosina [INN-Spanish]; Terazosine [INN-French]; Terazosinum [INN-Latin]; [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl](tetrahydrofuran-2-yl)methanone; [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(oxolan-2-yl)methanone; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-((tetrahydro-2-furanyl)carbonyl)piperazine; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(tetrahydro-2-furoyl)piperazine; 6,7-bis(methyloxy)-2-[4-(tetrahydrofuran-2-ylcarbonyl)piperazin-1-yl]quinazolin-4-amine; 6,7-dimethoxy-2-[4-(tetrahydrofuran-2-ylcarbonyl)piperazin-1-yl]quinazolin-4-amine
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Benign prostatic hyperplasia [ICD-11: GA90; ICD-10: N40; ICD-9: 600] | Approved | [1], [2] | |
| Therapeutic Class |
Anticancer Agents
|
|||
| Company |
Abbott Laboratories
|
|||
| Structure |
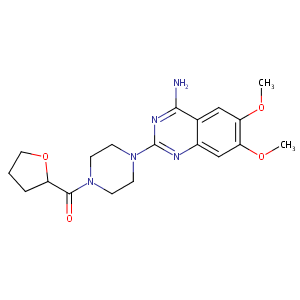 |
Download2D MOL |
||
| Formula |
C19H25N5O4
|
|||
| Canonical SMILES |
COC1=C(C=C2C(=C1)C(=NC(=N2)N3CCN(CC3)C(=O)C4CCCO4)N)OC
|
|||
| InChI |
1S/C19H25N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h10-11,14H,3-9H2,1-2H3,(H2,20,21,22)
|
|||
| InChIKey |
VCKUSRYTPJJLNI-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 63590-64-7
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9337, 855611, 3206271, 5639778, 7980757, 8153320, 11466779, 11467899, 11486437, 14805011, 29224453, 46509129, 47285410, 47583257, 47953972, 48104698, 48328570, 48416598, 49698629, 50011223, 50085853, 50104877, 56464178, 57322756, 79795430, 85209414, 85788394, 90340975, 92309317, 92710520, 92729635, 93625098, 96025253, 103185057, 103941452, 104170187, 104309137, 117762845, 121362449, 124750263, 124800293, 124881671, 124881672, 125325415, 125355205, 126631797, 126685615, 129455086, 131549269, 134337681
|
|||
| ChEBI ID |
CHEBI:9445
|
|||
| ADReCS Drug ID | BADD_D02158 ; BADD_D02159 | |||
| SuperDrug ATC ID |
G04CA03
|
|||
| SuperDrug CAS ID |
cas=063590647
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adrenergic receptor alpha-1D (ADRA1D) | Target Info | Antagonist | [3], [4] |
| KEGG Pathway | Calcium signaling pathway | |||
| cGMP-PKG signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Adrenergic signaling in cardiomyocytes | ||||
| Vascular smooth muscle contraction | ||||
| Salivary secretion | ||||
| NetPath Pathway | IL2 Signaling Pathway | |||
| Reactome | Adrenoceptors | |||
| G alpha (q) signalling events | ||||
| G alpha (12/13) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| Calcium Regulation in the Cardiac Cell | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7302). | |||
| REF 2 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 074823. | |||
| REF 3 | Induction of prostate apoptosis by alpha1-adrenoceptor antagonists: mechanistic significance of the quinazoline component. Prostate Cancer Prostatic Dis. 2002;5(2):88-95. | |||
| REF 4 | Overexpression of the alpha1B-adrenergic receptor causes apoptotic neurodegeneration: multiple system atrophy. Nat Med. 2000 Dec;6(12):1388-94. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

