Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Q6KO
|
|||
| Former ID |
DNC008837
|
|||
| Drug Name |
9-(2-Hydroxypropyl)-9H-adenine
|
|||
| Synonyms |
9-(2-Hydroxypropyl)adenine; 1-(6-aminopurin-9-yl)propan-2-ol; CHEMBL504495; MJZYTEBKXLVLMY-UHFFFAOYSA-N; 9-(2-Hydroxypropyl)-9H-adenine; AC1L1CKL; 9H-Purine-9-ethanol, 6-amino-.alpha.-methyl-; SCHEMBL904363; CTK6A8458; 9-(2-Hydroxy-1-propyl)adenine; BCP14341; BDBM50257055; 3-(6-Amino-9-purinyl)-2-propanol; AKOS010941288; TRA0093894; AN-7936; 1-(6-Amino-purin-9-yl)-propan-2-ol; SY022843; A3032; 4CH-024207; 4CH-018216; 1-(6-Amino-9H-purin-9-yl)-2-propanol #; MFCD07369451 (97+%)
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
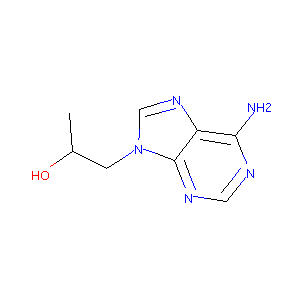 |
Download2D MOL |
||
| Formula |
C8H11N5O
|
|||
| Canonical SMILES |
CC(CN1C=NC2=C(N=CN=C21)N)O
|
|||
| InChI |
1S/C8H11N5O/c1-5(14)2-13-4-12-6-7(9)10-3-11-8(6)13/h3-5,14H,2H2,1H3,(H2,9,10,11)
|
|||
| InChIKey |
MJZYTEBKXLVLMY-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adenosine A2a receptor (ADORA2A) | Target Info | Inhibitor | [1] |
| KEGG Pathway | Rap1 signaling pathway | |||
| Calcium signaling pathway | ||||
| cAMP signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Vascular smooth muscle contraction | ||||
| Parkinson's disease | ||||
| Alcoholism | ||||
| Pathwhiz Pathway | Intracellular Signalling Through Adenosine Receptor A2a and Adenosine | |||
| Pathway Interaction Database | HIF-2-alpha transcription factor network | |||
| Reactome | NGF-independant TRKA activation | |||
| Adenosine P1 receptors | ||||
| G alpha (s) signalling events | ||||
| Surfactant metabolism | ||||
| WikiPathways | Nucleotide GPCRs | |||
| Monoamine Transport | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| NGF signalling via TRKA from the plasma membrane | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | 8-Bromo-9-alkyl adenine derivatives as tools for developing new adenosine A2A and A2B receptors ligands. Bioorg Med Chem. 2009 Apr 1;17(7):2812-22. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

