Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0S0AF
|
|||
| Former ID |
DIB001233
|
|||
| Drug Name |
Bunazosin
|
|||
| Synonyms |
Andante; E 015; DE-070; Detantol-R; E-1015; E-643
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Glaucoma/ocular hypertension [ICD-11: 9C61; ICD-9: 365] | Approved | [1] | |
| Company |
Eisai Co Ltd
|
|||
| Structure |
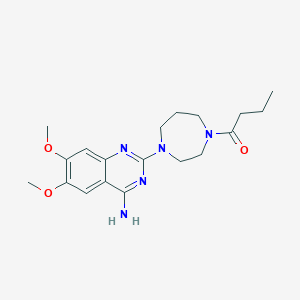 |
Download2D MOL |
||
| Formula |
C19H27N5O3
|
|||
| Canonical SMILES |
CCCC(=O)N1CCCN(CC1)C2=NC3=CC(=C(C=C3C(=N2)N)OC)OC
|
|||
| InChI |
1S/C19H27N5O3/c1-4-6-17(25)23-7-5-8-24(10-9-23)19-21-14-12-16(27-3)15(26-2)11-13(14)18(20)22-19/h11-12H,4-10H2,1-3H3,(H2,20,21,22)
|
|||
| InChIKey |
RHLJLALHBZGAFM-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 80755-51-7
|
|||
| PubChem Compound ID | ||||
| ChEBI ID |
CHEBI:135576
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Adrenergic receptor alpha-1D (ADRA1D) | Target Info | Modulator | [1], [2] |
| KEGG Pathway | Calcium signaling pathway | |||
| cGMP-PKG signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Adrenergic signaling in cardiomyocytes | ||||
| Vascular smooth muscle contraction | ||||
| Salivary secretion | ||||
| NetPath Pathway | IL2 Signaling Pathway | |||
| Reactome | Adrenoceptors | |||
| G alpha (q) signalling events | ||||
| G alpha (12/13) signalling events | ||||
| WikiPathways | Monoamine GPCRs | |||
| Calcium Regulation in the Cardiac Cell | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||
| REF 2 | Bunazosin, a selective alpha1-adrenoceptor antagonist, as an anti-glaucoma drug: effects on ocular circulation and retinal neuronal damage. Cardiovasc Drug Rev. 2005 Spring;23(1):43-56. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

