Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0S0RK
|
|||
| Former ID |
DIB019857
|
|||
| Drug Name |
flavone
|
|||
| Synonyms |
Flavone; FLAVONE; 525-82-6; 2-Phenylchromone; 2-Phenyl-4H-chromen-4-one; 2-Phenyl-4-chromone; Asmacoril; 2-Phenyl-4H-1-benzopyran-4-one; 2-Phenyl-4-benzopyron; 4H-1-Benzopyran-4-one, 2-phenyl-; Chromocor; Cromaril; 2-phenylchromen-4-one; Phenylchromone; 2-Phenyl-chromen-4-one; 2-Phenyl-gamma-benzopyrone; 2-Phenylbenzopyran-4-one; Cromarile; 2-Phenyl-4H-benzopyran-4-one; Flavon; 2-Phenylchrome; UNII-S2V45N7G3B; NSC 19028; 2-Phenyl-4H-chromen-4-on; CCRIS 4288; NSC19028; EINECS 208-383-8; NSC-19028; BRN 0157598
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1], [2] | |
| Structure |
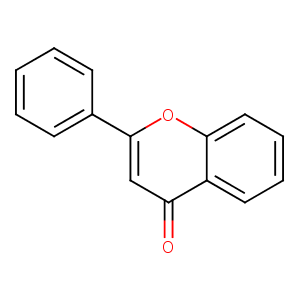 |
Download2D MOL |
||
| Formula |
C15H10O2
|
|||
| Canonical SMILES |
C1=CC=C(C=C1)C2=CC(=O)C3=CC=CC=C3O2
|
|||
| InChI |
1S/C15H10O2/c16-13-10-15(11-6-2-1-3-7-11)17-14-9-5-4-8-12(13)14/h1-10H
|
|||
| InChIKey |
VHBFFQKBGNRLFZ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 525-82-6
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
12229, 81975, 589074, 621065, 894248, 3133268, 5422192, 8138030, 8157719, 10322913, 10525272, 11145517, 11352088, 11393367, 14797981, 17137132, 17389570, 17396599, 24870087, 24894806, 26697253, 26752852, 29204142, 29229149, 30279698, 32626201, 46493766, 47515677, 47960105, 48185348, 48415240, 48421829, 48425145, 49737809, 49747452, 49889785, 50113345, 51075505, 53790304, 56462798, 57326386, 57393442, 57654180, 57807511, 79531197, 85086078, 85209887, 85293760, 85843922, 87569829
|
|||
| ChEBI ID |
CHEBI:42491
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 409). | |||
| REF 2 | Refinement and evaluation of a pharmacophore model for flavone derivatives binding to the benzodiazepine site of the GABA(A) receptor. J Med Chem. 2002 Sep 12;45(19):4188-201. | |||
| REF 3 | Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists. J Med Chem. 1996 Jun 7;39(12):2293-301. | |||
| REF 4 | Interactions of flavonoids and other phytochemicals with adenosine receptors. J Med Chem. 1996 Feb 2;39(3):781-8. | |||
| REF 5 | Effect of flavonoids on androgen and glucocorticoid receptors based on in vitro reporter gene assay. Bioorg Med Chem Lett. 2009 Aug 15;19(16):4706-10. | |||
| REF 6 | Pharmacophore modeling strategies for the development of novel nonsteroidal inhibitors of human aromatase (CYP19). Bioorg Med Chem Lett. 2010 May 15;20(10):3050-64. | |||
| REF 7 | Synthesis of halogenated/nitrated flavone derivatives and evaluation of their affinity for the central benzodiazepine receptor, Bioorg. Med. Chem. Lett. 7(15):2003-2008 (1997). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

