Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0S9YX
|
|||
| Former ID |
DIB018977
|
|||
| Drug Name |
biochanin A
|
|||
| Synonyms |
Biochanin A; 491-80-5; Biochanin; 4'-Methylgenistein; 5,7-Dihydroxy-4'-methoxyisoflavone; 5,7-Dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one; Genistein 4-methyl ether; 5,7-Dihydrox -4'-methoxyisoflavone; Biochanine A; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-methoxyphenyl)-; olmelin; Pratensol; NSC 123538; Biochanin-A; 5,7-dihydroxy-3-(4-methoxyphenyl)chromen-4-one; 4-Methylgenistein; C16H12O5; UNII-U13J6U390T; CCRIS 5449; 5,7-Dihydroxy-3-p-methoxyphenyl-4H-chromen-4-one; EINECS 207-744-7; NSC123538; Genistein 4'-methyl ether; 4'-methylgenistein; BIOCHANIN
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1], [2] | |
| Structure |
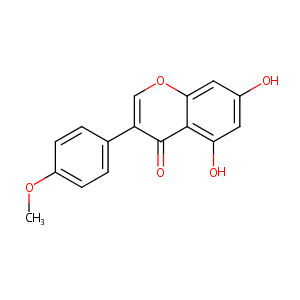 |
Download2D MOL |
||
| Formula |
C16H12O5
|
|||
| Canonical SMILES |
COC1=CC=C(C=C1)C2=COC3=CC(=CC(=C3C2=O)O)O
|
|||
| InChI |
1S/C16H12O5/c1-20-11-4-2-9(3-5-11)12-8-21-14-7-10(17)6-13(18)15(14)16(12)19/h2-8,17-18H,1H3
|
|||
| InChIKey |
WUADCCWRTIWANL-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 491-80-5
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
4072, 416963, 605430, 855738, 6896514, 8140097, 8144597, 8150182, 8616243, 11113288, 11336202, 11361441, 11363089, 11365651, 11368213, 11372095, 11375412, 11376375, 11404953, 11462413, 11483909, 11487863, 11490913, 11493741, 11494009, 14799798, 24893597, 26611560, 26679994, 26746976, 26751494, 29203891, 39289535, 46510178, 47365294, 47515401, 47589077, 47959856, 48334590, 48421900, 49684192, 49889542, 50100559, 50104034, 50123128, 50135482, 51062354, 53788092, 56422868, 57357716
|
|||
| ChEBI ID |
CHEBI:17574
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2829). | |||
| REF 2 | Inhibition of Plasmodium falciparum fatty acid biosynthesis: evaluation of FabG, FabZ, and FabI as drug targets for flavonoids. J Med Chem. 2006 Jun 1;49(11):3345-53. | |||
| REF 3 | Pharmacophore modeling strategies for the development of novel nonsteroidal inhibitors of human aromatase (CYP19). Bioorg Med Chem Lett. 2010 May 15;20(10):3050-64. | |||
| REF 4 | Flavone and isoflavone phytoestrogens are agonists of estrogen-related receptors. Mol Cancer Res. 2003 Nov;1(13):981-91. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

