Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0WL0Q
|
|||
| Former ID |
DNCL003555
|
|||
| Drug Name |
GS-5745
|
|||
| Indication | Gastric adenocarcinoma [ICD-11: 2B72; ICD-9: 151] | Phase 3 | [1] | |
| Ulcerative colitis [ICD-11: DD71; ICD-9: 556] | Phase 3 | [2] | ||
| Crohn disease [ICD-11: DD70; ICD-10: K50, K50.9; ICD-9: 555] | Phase 2 | [2] | ||
| Solid tumour/cancer [ICD-11: 2A00-2F9Z] | Phase 2 | [3] | ||
| Rheumatoid arthritis [ICD-11: FA20] | Phase 1 | [2] | ||
| Company |
GILEAD
|
|||
| Structure |
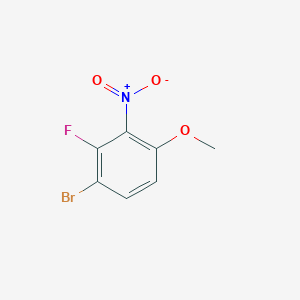 |
Download2D MOL |
||
| Formula |
C7H5BrFNO3
|
|||
| Canonical SMILES |
COC1=C(C(=C(C=C1)Br)F)[N+](=O)[O-]
|
|||
| InChI |
1S/C7H5BrFNO3/c1-13-5-3-2-4(8)6(9)7(5)10(11)12/h2-3H,1H3
|
|||
| InChIKey |
LJJAOMRFFXQENV-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 1352317-80-6
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 3 | ClinicalTrials.gov (NCT02405442) Safety and Efficacy of GS-5745 in Participants With Moderately to Severely Active Crohn's Disease. U.S. National Institutes of Health. | |||
| REF 4 | National Cancer Institute Drug Dictionary (drug id 747683). | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

