Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T54156
(Former ID: TTDC00076)
|
|||||
| Target Name |
Matrix metalloproteinase-9 (MMP-9)
|
|||||
| Synonyms |
Matrix metalloproteinase 9; GELB; CLG4B; 92 kDa type IV collagenase; 92 kDa gelatinase
Click to Show/Hide
|
|||||
| Gene Name |
MMP9
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 4 Target-related Diseases | + | ||||
| 1 | Ischaemic/haemorrhagic stroke [ICD-11: 8B20] | |||||
| 2 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| 3 | Stomach cancer [ICD-11: 2B72] | |||||
| 4 | Ulcerative colitis [ICD-11: DD71] | |||||
| Function |
Could play a role in bone osteoclastic resorption. Cleaves KiSS1 at a Gly-|-Leu bond. Cleaves type IV and type V collagen into large C-terminal three quarter fragments and shorter N-terminal one quarter fragments. Degrades fibronectin but not laminin or Pz-peptide. May play an essential role in local proteolysis of the extracellular matrix and in leukocyte migration.
Click to Show/Hide
|
|||||
| BioChemical Class |
Peptidase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.4.24.35
|
|||||
| Sequence |
MSLWQPLVLVLLVLGCCFAAPRQRQSTLVLFPGDLRTNLTDRQLAEEYLYRYGYTRVAEM
RGESKSLGPALLLLQKQLSLPETGELDSATLKAMRTPRCGVPDLGRFQTFEGDLKWHHHN ITYWIQNYSEDLPRAVIDDAFARAFALWSAVTPLTFTRVYSRDADIVIQFGVAEHGDGYP FDGKDGLLAHAFPPGPGIQGDAHFDDDELWSLGKGVVVPTRFGNADGAACHFPFIFEGRS YSACTTDGRSDGLPWCSTTANYDTDDRFGFCPSERLYTQDGNADGKPCQFPFIFQGQSYS ACTTDGRSDGYRWCATTANYDRDKLFGFCPTRADSTVMGGNSAGELCVFPFTFLGKEYST CTSEGRGDGRLWCATTSNFDSDKKWGFCPDQGYSLFLVAAHEFGHALGLDHSSVPEALMY PMYRFTEGPPLHKDDVNGIRHLYGPRPEPEPRPPTTTTPQPTAPPTVCPTGPPTVHPSER PTAGPTGPPSAGPTGPPTAGPSTATTVPLSPVDDACNVNIFDAIAEIGNQLYLFKDGKYW RFSEGRGSRPQGPFLIADKWPALPRKLDSVFEERLSKKLFFFSGRQVWVYTGASVLGPRR LDKLGLGADVAQVTGALRSGRGKMLLFSGRRLWRFDVKAQMVDPRSASEVDRMFPGVPLD THDVFQYREKAYFCQDRFYWRVSSRSELNQVDQVGYVTYDILQCPED Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T49TDM | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 6 Clinical Trial Drugs | + | ||||
| 1 | Andecaliximab | Drug Info | Phase 3 | Gastric adenocarcinoma | [2] | |
| 2 | Curcumin | Drug Info | Phase 3 | Solid tumour/cancer | [3], [4] | |
| 3 | DP-b99 | Drug Info | Phase 3 | Stroke | [5] | |
| 4 | GS-5745 | Drug Info | Phase 3 | Gastric adenocarcinoma | [6] | |
| 5 | BLZ-100 | Drug Info | Phase 1/2 | Glioma | [7] | |
| 6 | Neovastat | Drug Info | Phase 1 | Renal cell carcinoma | [8] | |
| Discontinued Drug(s) | [+] 6 Discontinued Drugs | + | ||||
| 1 | Galarubicin | Drug Info | Discontinued in Phase 2 | Solid tumour/cancer | [9] | |
| 2 | GM6001 | Drug Info | Discontinued in Phase 2 | Corneal ulcer | [10] | |
| 3 | RS-130830 | Drug Info | Discontinued in Phase 2 | Hepatitis C virus infection | [11] | |
| 4 | CDP-845 | Drug Info | Terminated | Solid tumour/cancer | [13] | |
| 5 | CT-1746 | Drug Info | Terminated | Colorectal cancer | [13] | |
| 6 | RO-319790 | Drug Info | Terminated | Rheumatoid arthritis | [14] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | DX-2802 | Drug Info | Preclinical | Solid tumour/cancer | [12] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 51 Inhibitor drugs | + | ||||
| 1 | Curcumin | Drug Info | [1] | |||
| 2 | DP-b99 | Drug Info | [16] | |||
| 3 | BLZ-100 | Drug Info | [7] | |||
| 4 | Neovastat | Drug Info | [18], [19], [20] | |||
| 5 | PMID29130358-Compound-Figure13(4) | Drug Info | [21] | |||
| 6 | PMID29130358-Compound-Figure17(10) | Drug Info | [21] | |||
| 7 | PMID29130358-Compound-Figure17(11) | Drug Info | [21] | |||
| 8 | PMID29130358-Compound-Figure17(12) | Drug Info | [21] | |||
| 9 | PMID29130358-Compound-Figure17(13) | Drug Info | [21] | |||
| 10 | PMID29130358-Compound-Figure18(14a) | Drug Info | [21] | |||
| 11 | PMID29130358-Compound-LonimacranthoideVII | Drug Info | [21] | |||
| 12 | PMID29130358-Compound-SB-3CT | Drug Info | [21] | |||
| 13 | Galarubicin | Drug Info | [22] | |||
| 14 | GM6001 | Drug Info | [23] | |||
| 15 | RS-130830 | Drug Info | [24] | |||
| 16 | RO-319790 | Drug Info | [28] | |||
| 17 | SC-44463 | Drug Info | [29] | |||
| 18 | (+/-)5-(biphenyl-4-yl)-3-hydroxypentanoic acid | Drug Info | [30] | |||
| 19 | 2-(4'-chloro-biphenyl-4-sulfonyl)-pentanoic acid | Drug Info | [31] | |||
| 20 | 2-(biphenyl-4-ylsulfonamido)pentanedioic acid | Drug Info | [32] | |||
| 21 | 2-(Biphenyl-4-ylsulfonyl)N-hydroxybenzamide | Drug Info | [33] | |||
| 22 | 2-Amino-N,3,3-Trimethylbutanamide | Drug Info | [34] | |||
| 23 | 3-(4-(2-phenylethynyl)benzoyl)pentanoic acid | Drug Info | [35] | |||
| 24 | 3-(4-Phenylethynylbenzoyl)nonanoic acid | Drug Info | [35] | |||
| 25 | 4-amino-3-(4-(hexyloxy)phenyl)-4-oxobutanoic acid | Drug Info | [32] | |||
| 26 | 5-(4-Phenoxy-phenyl)-pyrimidine-2,4,6-trione | Drug Info | [36] | |||
| 27 | 5-Biphenyl-4-yl-5-ethyl-pyrimidine-2,4,6-trione | Drug Info | [36] | |||
| 28 | 5-Biphenyl-4-yl-5-hexyl-pyrimidine-2,4,6-trione | Drug Info | [36] | |||
| 29 | 5-Hexyl-5-phenyl-pyrimidine-2,4,6-trione | Drug Info | [36] | |||
| 30 | 5-Methyl-5-phenyl-pyrimidine-2,4,6-trione | Drug Info | [36] | |||
| 31 | ARP100 | Drug Info | [37] | |||
| 32 | Carboxylated glucosamine | Drug Info | [38] | |||
| 33 | Folate gamma-hydroxamic acid | Drug Info | [39] | |||
| 34 | IK-862 | Drug Info | [40] | |||
| 35 | Methotrexate gamma-hydroxamic acid | Drug Info | [39] | |||
| 36 | Methotrexate gamma-L-phenylalaninehydroxamic acid | Drug Info | [39] | |||
| 37 | Methotrexate gamma-L-proline-hydroxamic acid | Drug Info | [39] | |||
| 38 | MMI270 | Drug Info | [41] | |||
| 39 | N-hydroxy-2,3-bis(phenylsulfonamido)propanamide | Drug Info | [42] | |||
| 40 | N-Hydroxy-2-(4-phenoxy-benzenesulfonyl)benzamide | Drug Info | [33] | |||
| 41 | N-hydroxy-3-(2-oxo-2H-chromen-3-yl)propanamide | Drug Info | [43] | |||
| 42 | N-hydroxy-3-(6-methoxy-2-oxo-2H-chromen-3-yl) | Drug Info | [43] | |||
| 43 | PMID15055993C1a | Drug Info | [44] | |||
| 44 | PMID23631440C29e | Drug Info | [45] | |||
| 45 | Ro 28-2653 | Drug Info | [46] | |||
| 46 | Ro-37-9790 | Drug Info | [47] | |||
| 47 | Roche 28-2653 | Drug Info | [46] | |||
| 48 | SL422 | Drug Info | [48] | |||
| 49 | SR-973 | Drug Info | [49] | |||
| 50 | UK-356618 | Drug Info | [50] | |||
| 51 | [2-(Biphenyl-4-sulfonyl)phenyl]acetic Acid | Drug Info | [33] | |||
| Modulator | [+] 3 Modulator drugs | + | ||||
| 1 | GS-5745 | Drug Info | [17] | |||
| 2 | CDP-845 | Drug Info | [26] | |||
| 3 | CT-1746 | Drug Info | [27] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Piperazine | Ligand Info | |||||
| Structure Description | Crystal structure of MMP9 in complex with inhibitor BE4. | PDB:6ESM | ||||
| Method | X-ray diffraction | Resolution | 1.10 Å | Mutation | Yes | [51] |
| PDB Sequence |
GDLKWHHHNI
121 TYWIQNYSED131 LPRAVIDDAF141 ARAFALWSAV151 TPLTFTRVYS161 RDADIVIQFG 171 VAEHGDGYPF181 DGKDGLLAHA191 FPPGPGIQGD201 AHFDDDELWS211 LGKGVGYSLF 221 LVAAHQFGHA231 LGLDHSSVPE241 ALMYPMYRFT251 EGPPLHKDDV261 NGIRHLYG |
|||||
|
|
||||||
| Ligand Name: Cobalt Hexammine Ion | Ligand Info | |||||
| Structure Description | Structure determination of a potent, selective antibody inhibitor of human MMP9 (GS-5745 bound to MMP-9) | PDB:5TH9 | ||||
| Method | X-ray diffraction | Resolution | 3.00 Å | Mutation | No | [52] |
| PDB Sequence |
TDRQLAEEYL
49 YRYGYTRVAS66 LGPALLLLQK76 QLSLPETGEL86 DSATLKAMRT96 PRCGVPDLGR 106 FQTFEGDLKW116 HHHNITYWIQ126 NYSEDLPRAV136 IDDAFARAFA146 LWSAVTPLTF 156 TRVYSRDADI166 VIQFGVAEHG176 DGYPFDGKDG186 LLAHAFPPGP196 GIQGDAHFDD 206 DELWSLGKGQ391 GYSLFLVAAH401 EFGHALGLDH411 SSVPEALMYP421 MYRFTEGPPL 431 HKDDVNGIRH441 LY
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Microtubule-actin cross-linking factor 1, isoforms 6/7 (MACF1) | 36.709 (29/79) | 4.06E-04 |
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
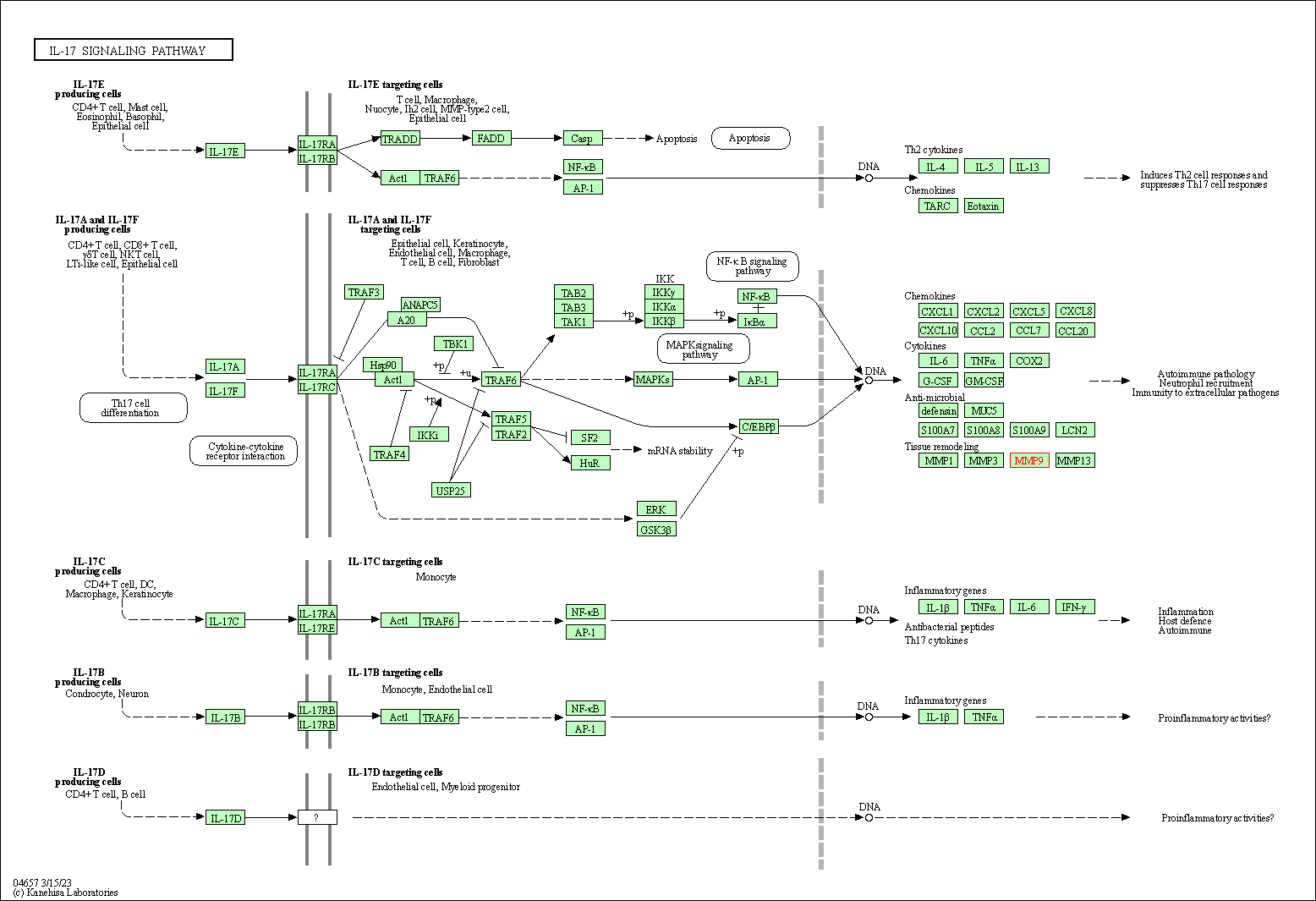
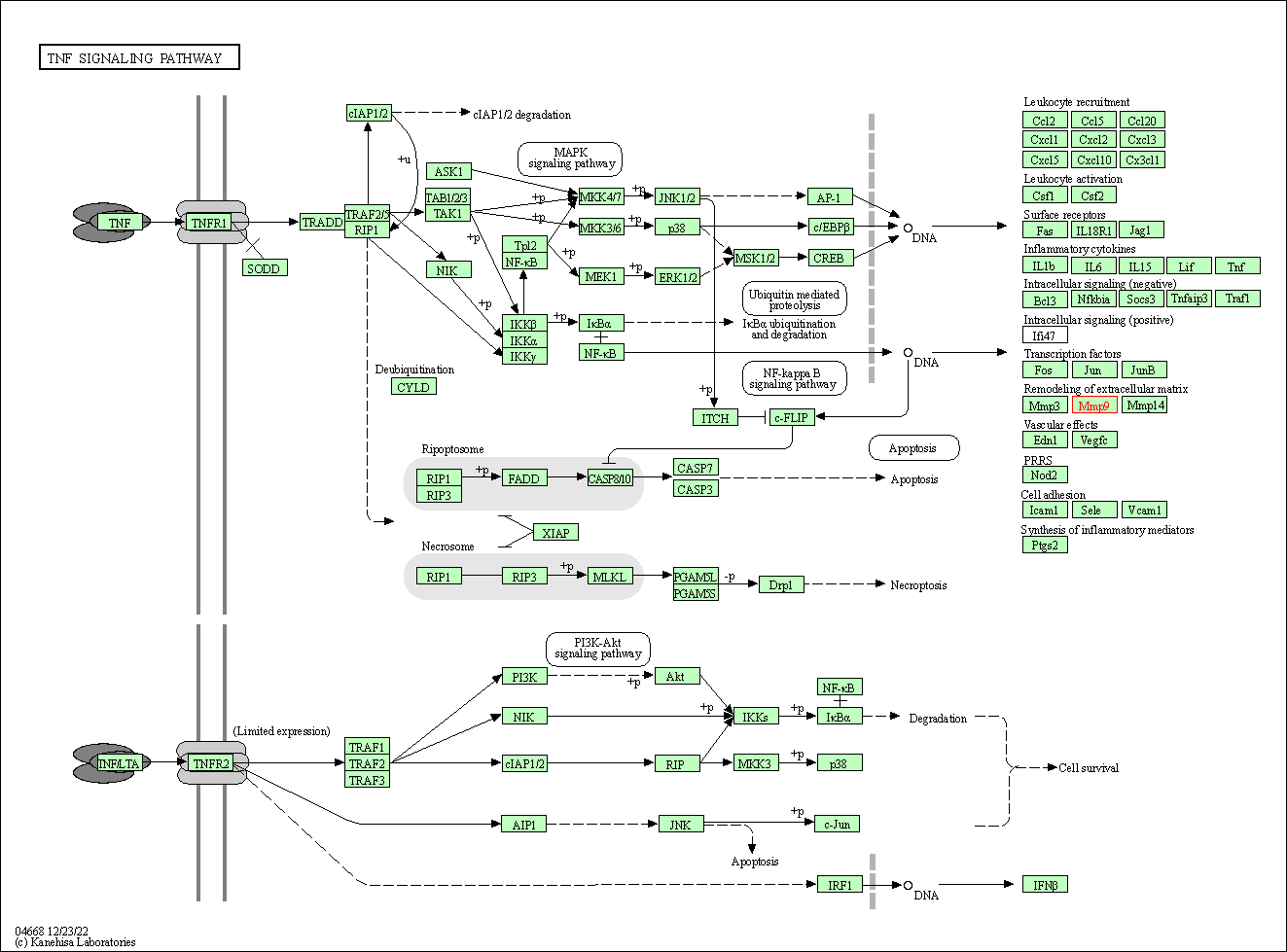
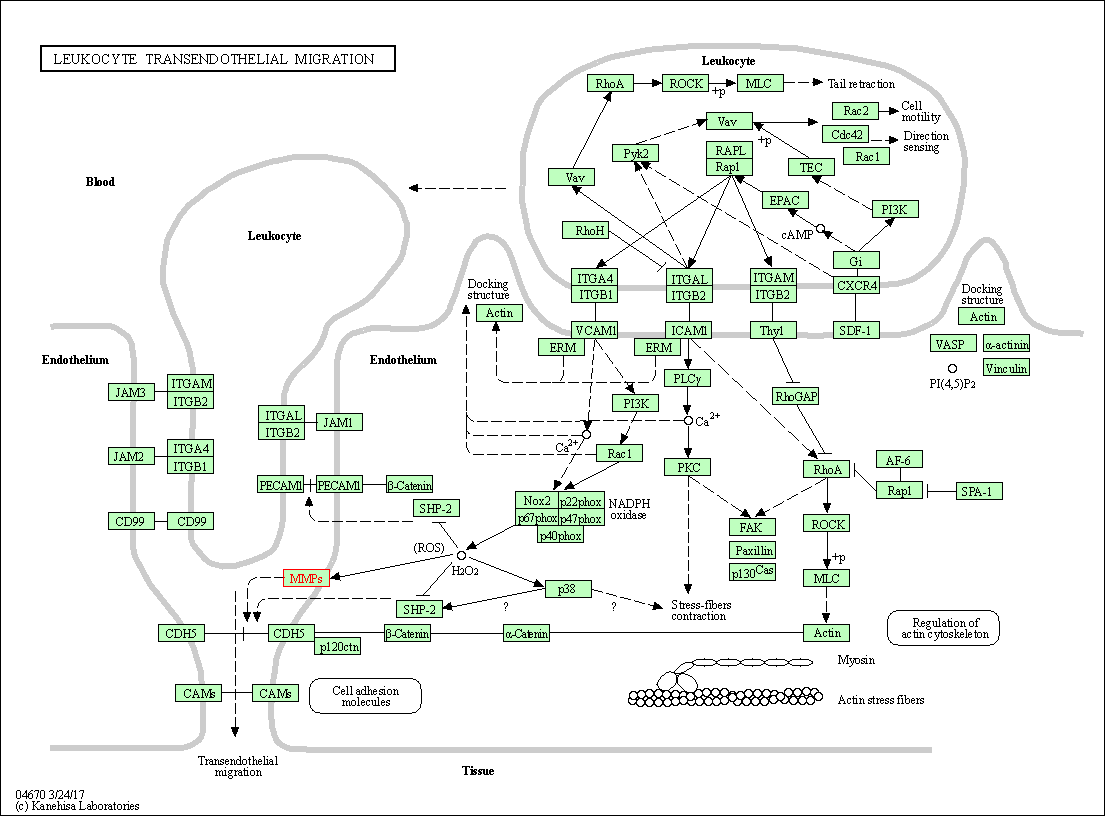
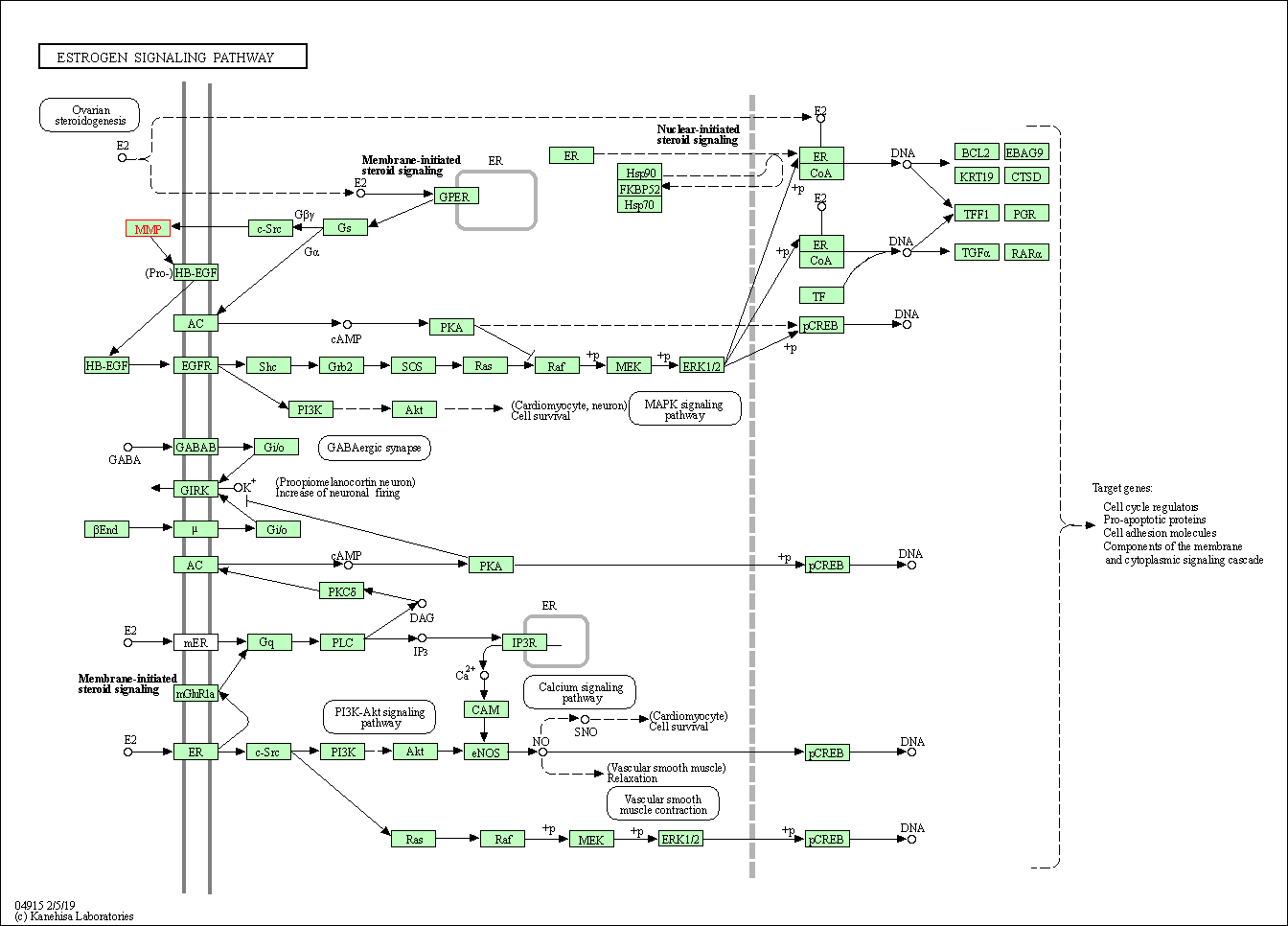
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| IL-17 signaling pathway | hsa04657 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| TNF signaling pathway | hsa04668 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Leukocyte transendothelial migration | hsa04670 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Estrogen signaling pathway | hsa04915 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
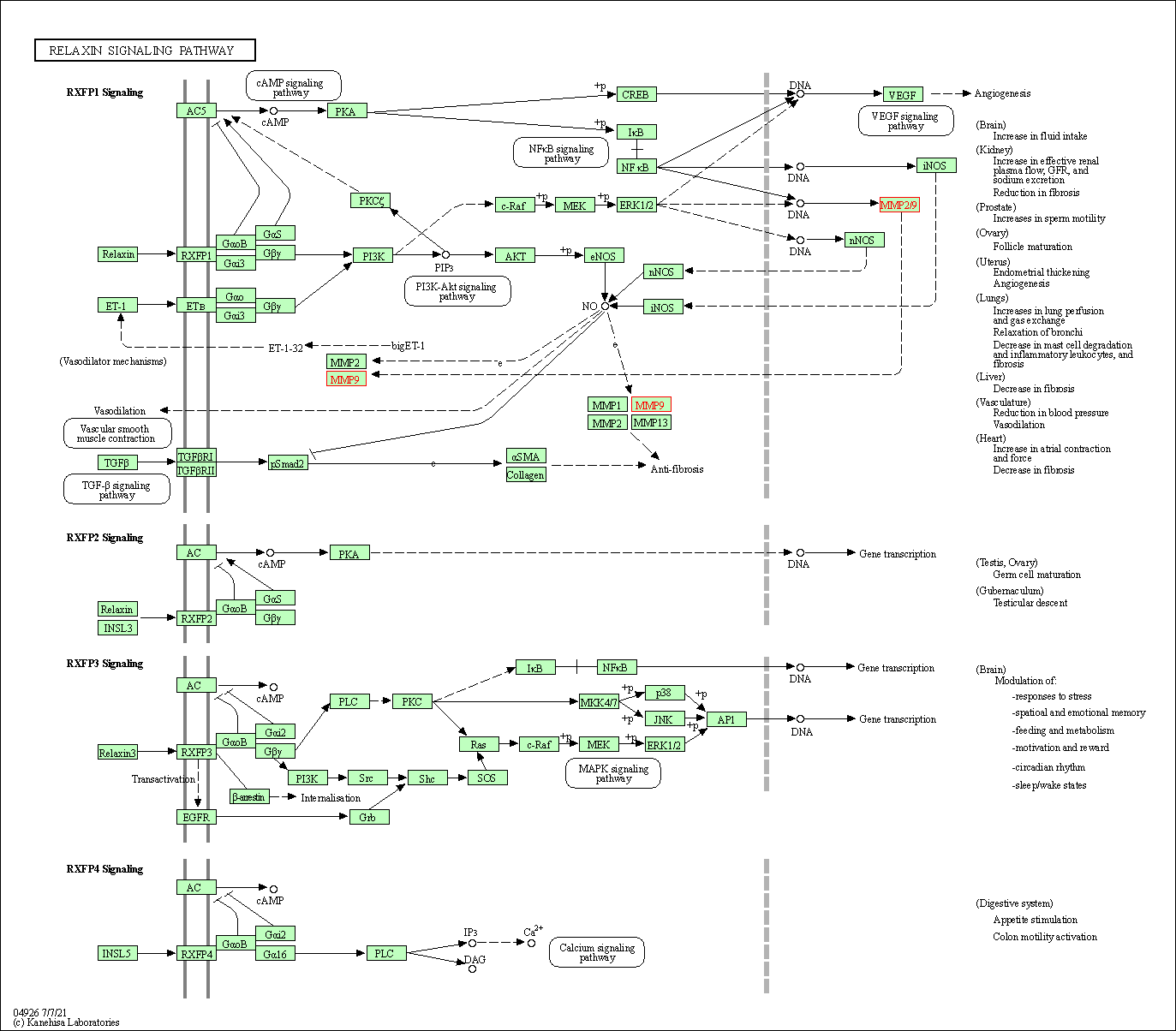
| Relaxin signaling pathway | hsa04926 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 25 | Degree centrality | 2.69E-03 | Betweenness centrality | 1.36E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.40E-01 | Radiality | 1.42E+01 | Clustering coefficient | 2.13E-01 |
| Neighborhood connectivity | 3.56E+01 | Topological coefficient | 7.07E-02 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Synthesis and biological evaluation of curcuminoid pyrazoles as new therapeutic agents in inflammatory bowel disease: effect on matrix metalloprote... Bioorg Med Chem. 2009 Feb 1;17(3):1290-6. | |||||
| REF 2 | ClinicalTrials.gov (NCT02545504) Andecaliximab With mFOLFOX6 as First Line Treatment for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma (GAMMA-1). U.S. National Institutes of Health. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7000). | |||||
| REF 4 | Nanocurcumin: a promising therapeutic advancement over native curcumin. Crit Rev Ther Drug Carrier Syst. 2013;30(4):331-68. | |||||
| REF 5 | ClinicalTrials.gov (NCT00893867) Efficacy and Safety Study of DP-b99 in Treating Acute Ischemic Stroke. U.S. National Institutes of Health. | |||||
| REF 6 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 7 | Antibodies and venom peptides: new modalities for ion channels. Nat Rev Drug Discov. 2019 May;18(5):339-357. | |||||
| REF 8 | Emerging therapies for multiple myeloma. Expert Opin Emerg Drugs. 2009 Mar;14(1):99-127. | |||||
| REF 9 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004410) | |||||
| REF 10 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001387) | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010620) | |||||
| REF 12 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800024278) | |||||
| REF 13 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006498) | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002350) | |||||
| REF 15 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 16 | DP-b99 modulates matrix metalloproteinase activity and neuronal plasticity. PLoS One. 2014 Jun 11;9(6):e99789. | |||||
| REF 17 | National Cancer Institute Drug Dictionary (drug id 747683). | |||||
| REF 18 | Neovastat, a naturally occurring multifunctional antiangiogenic drug, in phase III clinical trials. Semin Oncol. 2001 Dec;28(6):620-5. | |||||
| REF 19 | Neovastat (AE-941) inhibits the airway inflammation and hyperresponsiveness in a murine model of asthma. J Microbiol. 2005 Feb;43(1):11-6. | |||||
| REF 20 | The effect of Neovastat (AE-941) on an experimental metastatic bone tumor model. Int J Oncol. 2002 Feb;20(2):299-303. | |||||
| REF 21 | Gelatinase inhibitors: a patent review (2011-2017).Expert Opin Ther Pat. 2018 Jan;28(1):31-46. | |||||
| REF 22 | Inhibitory effect of DA-125, a new anthracyclin analog antitumor agent, on the invasion of human fibrosarcoma cells by down-regulating the matrix m... Biochem Pharmacol. 2005 Dec 19;71(1-2):21-31. | |||||
| REF 23 | Introduction of the 4-(4-bromophenyl)benzenesulfonyl group to hydrazide analogs of Ilomastat leads to potent gelatinase B (MMP-9) inhibitors with i... Bioorg Med Chem. 2008 Sep 15;16(18):8745-59. | |||||
| REF 24 | Structure-based design of potent and selective inhibitors of collagenase-3 (MMP-13). Bioorg Med Chem Lett. 2005 Feb 15;15(4):1101-6. | |||||
| REF 25 | Clinical pipeline report, company report or official report of DeepDyve. | |||||
| REF 26 | Clinical potential of matrix metalloprotease inhibitors. Drugs R D. 1999 Feb;1(2):117-29. | |||||
| REF 27 | Conversion of highly malignant colon cancer from an aggressive to a controlled disease by oral administration of a metalloproteinase inhibitor. Clin Exp Metastasis. 1997 Mar;15(2):184-95. | |||||
| REF 28 | The asymmetric synthesis and in vitro characterization of succinyl mercaptoalcohol and mercaptoketone inhibitors of matrix metalloproteinases. Bioorg Med Chem Lett. 1998 May 19;8(10):1163-8. | |||||
| REF 29 | Design and synthesis of a series of (2R)-N(4)-hydroxy-2-(3-hydroxybenzyl)-N(1)- [(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]butanediamide derivati... J Med Chem. 2001 Oct 11;44(21):3347-50. | |||||
| REF 30 | The identification of beta-hydroxy carboxylic acids as selective MMP-12 inhibitors. Bioorg Med Chem Lett. 2009 Oct 1;19(19):5760-3. | |||||
| REF 31 | Synthesis and SAR of alpha-sulfonylcarboxylic acids as potent matrix metalloproteinase inhibitors. Bioorg Med Chem Lett. 2006 Jun 15;16(12):3096-100. | |||||
| REF 32 | Ranking the selectivity of PubChem screening hits by activity-based protein profiling: MMP13 as a case study. Bioorg Med Chem. 2009 Feb 1;17(3):1101-8. | |||||
| REF 33 | Design, synthesis, biological evaluation, and NMR studies of a new series of arylsulfones as selective and potent matrix metalloproteinase-12 inhib... J Med Chem. 2009 Oct 22;52(20):6347-61. | |||||
| REF 34 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 35 | Selective inhibition of matrix metalloproteinase isozymes and in vivo protection against emphysema by substituted gamma-keto carboxylic acids. J Med Chem. 2006 Jan 26;49(2):456-8. | |||||
| REF 36 | Novel 5,5-disubstitutedpyrimidine-2,4,6-triones as selective MMP inhibitors. Bioorg Med Chem Lett. 2001 Apr 23;11(8):969-72. | |||||
| REF 37 | Amber force field implementation, molecular modelling study, synthesis and MMP-1/MMP-2 inhibition profile of (R)- and (S)-N-hydroxy-2-(N-isopropoxy... Bioorg Med Chem. 2006 Jun 15;14(12):4260-76. | |||||
| REF 38 | Carboxy derivatized glucosamine is a potent inhibitor of matrix metalloproteinase-9 in HT1080 cells. Bioorg Med Chem Lett. 2006 Jun 15;16(12):3105-10. | |||||
| REF 39 | Methotrexate gamma-hydroxamate derivatives as potential dual target antitumor drugs. Bioorg Med Chem. 2007 Feb 1;15(3):1266-74. | |||||
| REF 40 | Discovery of gamma-lactam hydroxamic acids as selective inhibitors of tumor necrosis factor alpha converting enzyme: design, synthesis, and structu... J Med Chem. 2002 Nov 7;45(23):4954-7. | |||||
| REF 41 | Picking the S1, S1' and S2' pockets of matrix metalloproteinases. A niche for potent acyclic sulfonamide inhibitors. Bioorg Med Chem Lett. 1999 Jun 21;9(12):1691-6. | |||||
| REF 42 | Novel bis-(arylsulfonamide) hydroxamate-based selective MMP inhibitors. Bioorg Med Chem Lett. 2008 Jun 1;18(11):3333-7. | |||||
| REF 43 | Chromen-based TNF-alpha converting enzyme (TACE) inhibitors: design, synthesis, and biological evaluation. Bioorg Med Chem. 2008 Jan 1;16(1):530-5. | |||||
| REF 44 | Azasugar-based MMP/ADAM inhibitors as antipsoriatic agents. J Med Chem. 2004 Apr 8;47(8):1930-8. | |||||
| REF 45 | Matrix metalloproteinase inhibitors based on the 3-mercaptopyrrolidine core. J Med Chem. 2013 Jun 13;56(11):4357-73. | |||||
| REF 46 | The new synthetic matrix metalloproteinase inhibitor (Roche 28-2653) reduces tumor growth and prolongs survival in a prostate cancer standard rat model. Oncogene. 2002 Mar 27;21(13):2089-96. | |||||
| REF 47 | 11,21-Bisphenyl-19-norpregnane derivatives are selective antiglucocorticoids, Bioorg. Med. Chem. Lett. 7(17):2299-2302 (1997). | |||||
| REF 48 | Design, synthesis, and structure-activity relationships of macrocyclic hydroxamic acids that inhibit tumor necrosis factor alpha release in vitro and in vivo. J Med Chem. 2001 Aug 2;44(16):2636-60. | |||||
| REF 49 | Synthesis and evaluation of succinoyl-caprolactam gamma-secretase inhibitors. Bioorg Med Chem Lett. 2006 May 1;16(9):2357-63. | |||||
| REF 50 | A potent, selective inhibitor of matrix metalloproteinase-3 for the topical treatment of chronic dermal ulcers. J Med Chem. 2003 Jul 31;46(16):3514-25. | |||||
| REF 51 | Development of Thioaryl-Based Matrix Metalloproteinase-12 Inhibitors with Alternative Zinc-Binding Groups: Synthesis, Potentiometric, NMR, and Crystallographic Studies. J Med Chem. 2018 May 24;61(10):4421-4435. | |||||
| REF 52 | Biochemical characterization and structure determination of a potent, selective antibody inhibitor of human MMP9. J Biol Chem. 2017 Apr 21;292(16):6810-6820. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

