Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0WX6R
|
|||
| Former ID |
DNC007852
|
|||
| Drug Name |
5,6-dinitroacenaphthoquinone
|
|||
| Synonyms |
5,6-dinitroacenaphthoquinone; 5,6-dinitroacenaphthylene-1,2-dione; AC1MQHVL; 5,6-dinitroacenaphthenequinone; CHEMBL235289; SCHEMBL2699746; BDBM22854; MolPort-003-895-771; 1,2-Dione-Based Compound, 11; AKOS024343013; MCULE-5132922926; 27471-02-9; 5,6-dinitro-1,2-dihydroacenaphthylenedione
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
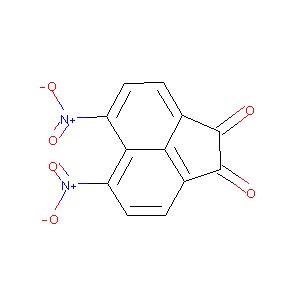 |
Download2D MOL |
||
| Formula |
C12H4N2O6
|
|||
| Canonical SMILES |
C1=CC(=C2C(=CC=C3C2=C1C(=O)C3=O)[N+](=O)[O-])[N+](=O)[O-]
|
|||
| InChI |
1S/C12H4N2O6/c15-11-5-1-3-7(13(17)18)10-8(14(19)20)4-2-6(9(5)10)12(11)16/h1-4H
|
|||
| InChIKey |
HKNXJYVCBQHLBT-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Acetylcholinesterase (AChE) | Target Info | Inhibitor | [1] |
| Cholinesterase (BCHE) | Target Info | Inhibitor | [1] | |
| Liver carboxylesterase (CES1) | Target Info | Inhibitor | [1] | |
| KEGG Pathway | Glycerophospholipid metabolism | |||
| Cholinergic synapse | ||||
| Drug metabolism - other enzymes | ||||
| Metabolic pathways | ||||
| Panther Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | |||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | ||||
| Nicotinic acetylcholine receptor signaling pathway | ||||
| Pathwhiz Pathway | Phospholipid Biosynthesis | |||
| Pathway Interaction Database | ATF-2 transcription factor network | |||
| E2F transcription factor network | ||||
| WikiPathways | Irinotecan Pathway | |||
| Monoamine Transport | ||||
| Biogenic Amine Synthesis | ||||
| Acetylcholine Synthesis | ||||
| Integrated Pancreatic Cancer Pathway | ||||
| NRF2 pathway | ||||
| Nuclear Receptors Meta-Pathway | ||||
| Heroin metabolism | ||||
| Fluoropyrimidine Activity | ||||
| Phase I biotransformations, non P450 | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Planarity and constraint of the carbonyl groups in 1,2-diones are determinants for selective inhibition of human carboxylesterase 1. J Med Chem. 2007 Nov 15;50(23):5727-34. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

