Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Y0OA
|
|||
| Former ID |
DNC006449
|
|||
| Drug Name |
Ochnaflavone
|
|||
| Synonyms |
Ochnaflavone; 50276-96-5; Ochnaflavone7-O-beta-D-gluco-pyranoside; AC1NUSW2; SCHEMBL13829452; DTXSID70198281; ZINC28462577; 6882AB; AKOS016010710; AK120364; KB-282683; AX8245780; 4CH-024764; 2-[4-[5-(5,7-dihydroxy-4-oxochromen-2-yl)-2-hydroxyphenoxy]phenyl]-5,7-dihydroxychromen-4-one; 2-{4-[5-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)-2-hydroxyphenoxy]phenyl}-5,7-dihydroxy-4H-chromen-4-one; 4H-1-Benzopyran-4-one, 2-(4-(5-(5,7-dihydroxy-4-oxo-4H-1-benzopyran-2-yl)-2-hydroxyphenoxy)phenyl)-5,7-dihydroxy-
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
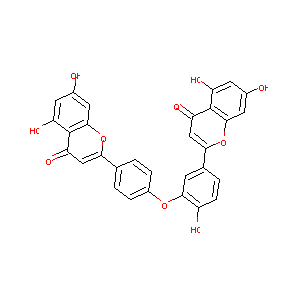 |
Download2D MOL |
||
| Formula |
C30H18O10
|
|||
| Canonical SMILES |
C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)OC4=C(C=CC(=C4)C5=CC(=O)C6=C(C=C(C=C6O5)O)O)O
|
|||
| InChI |
1S/C30H18O10/c31-16-8-20(34)29-22(36)12-24(39-27(29)10-16)14-1-4-18(5-2-14)38-26-7-15(3-6-19(26)33)25-13-23(37)30-21(35)9-17(32)11-28(30)40-25/h1-13,31-35H
|
|||
| InChIKey |
NNPGECDACGBKDH-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 50276-96-5
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Group IIA phospholipase A2 (GIIA sPLA2) | Target Info | Inhibitor | [1] |
| BioCyc | Phospholipases | |||
| KEGG Pathway | Glycerophospholipid metabolism | |||
| Ether lipid metabolism | ||||
| Arachidonic acid metabolism | ||||
| Linoleic acid metabolism | ||||
| alpha-Linolenic acid metabolism | ||||
| Metabolic pathways | ||||
| Ras signaling pathway | ||||
| Vascular smooth muscle contraction | ||||
| Pancreatic secretion | ||||
| Fat digestion and absorption | ||||
| Pathway Interaction Database | Glypican 1 network | |||
| Reactome | Acyl chain remodelling of PC | |||
| Acyl chain remodelling of PE | ||||
| Acyl chain remodelling of PI | ||||
| WikiPathways | Cardiac Hypertrophic Response | |||
| Glycerophospholipid biosynthesis | ||||
| Glycerophospholipid Biosynthetic Pathway | ||||
| Spinal Cord Injury | ||||
| Eicosanoid Synthesis | ||||
| MicroRNAs in cardiomyocyte hypertrophy | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Synthesis of phospholipase A2 inhibitory biflavonoids. Bioorg Med Chem Lett. 2006 May 1;16(9):2373-5. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

