Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Y6UU
|
|||
| Former ID |
DCL000296
|
|||
| Drug Name |
Atrasentan
|
|||
| Synonyms |
Xinlay; Atrasentan [INN]; A 127722; A 147627; ABT 627; A-127722; A-147627; ABT-627; Xinlay (TN); A-127722.5; (+)-A 127722; (+)-A-127722; (11C)ABT-627; (2R,3R,4S)-4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic acid; 2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(((dibutylamino)carbonyl)methyl)pyrrolidine-3-carboxylic acid; 2R-(4-methoxyphenyl)-4S(1,3-benzodioxol-5-yl)-1-(N,N-di(n-butyl)aminocarbonyl-methyl)-pyrrolidine-3R-carboxylic acid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Diabetic nephropathy [ICD-11: GB61.Z; ICD-9: 250.4] | Phase 3 | [1] | |
| Prostate cancer [ICD-11: 2C82.0] | Withdrawn from market | [2], [3] | ||
| Company |
Abbott
|
|||
| Structure |
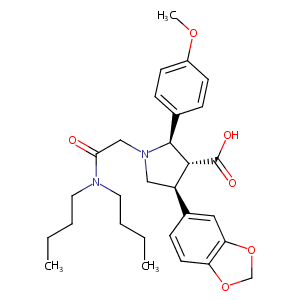 |
Download2D MOL |
||
| Formula |
C29H38N2O6
|
|||
| Canonical SMILES |
CCCCN(CCCC)C(=O)CN1CC(C(C1C2=CC=C(C=C2)OC)C(=O)O)C3=CC4=C(C=C3)OCO4
|
|||
| InChI |
1S/C29H38N2O6/c1-4-6-14-30(15-7-5-2)26(32)18-31-17-23(21-10-13-24-25(16-21)37-19-36-24)27(29(33)34)28(31)20-8-11-22(35-3)12-9-20/h8-13,16,23,27-28H,4-7,14-15,17-19H2,1-3H3,(H,33,34)/t23-,27-,28+/m1/s1
|
|||
| InChIKey |
MOTJMGVDPWRKOC-QPVYNBJUSA-N
|
|||
| CAS Number |
CAS 173937-91-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
535748, 10253652, 12014999, 14811316, 14909273, 46234806, 50068170, 50253083, 53788137, 53789849, 57348721, 103168476, 103948098, 113442732, 127346318, 128626310, 134338952, 134339653, 135115498, 135325628, 137006712, 141972835, 163620866, 163686195, 178100477, 179150068, 185974011, 198977266, 224005477, 226421083
|
|||
| ChEBI ID |
CHEBI:135810
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Endothelin A receptor (EDNRA) | Target Info | Antagonist | [4] |
| KEGG Pathway | Calcium signaling pathway | |||
| cGMP-PKG signaling pathway | ||||
| cAMP signaling pathway | ||||
| Neuroactive ligand-receptor interaction | ||||
| Vascular smooth muscle contraction | ||||
| Renin secretion | ||||
| Pathways in cancer | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Panther Pathway | Endothelin signaling pathway | |||
| Pathway Interaction Database | Endothelins | |||
| EGFR-dependent Endothelin signaling events | ||||
| Reactome | Peptide ligand-binding receptors | |||
| G alpha (q) signalling events | ||||
| WikiPathways | Prostaglandin Synthesis and Regulation | |||
| GPCRs, Class A Rhodopsin-like | ||||
| Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Peptide GPCRs | ||||
| Endothelin Pathways | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| GPCRs, Other | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3487). | |||
| REF 3 | Current and future treatments of bone metastases. Expert Opin Emerg Drugs. 2008 Dec;13(4):609-27. | |||
| REF 4 | Effects of a selective ET(A)-receptor antagonist, atrasentan (ABT-627), in murine models of allergic asthma: demonstration of mouse strain specificity. Clin Sci (Lond). 2002 Aug;103 Suppl 48:367S-370S. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

