Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T23499
(Former ID: TTDS00198)
|
|||||
| Target Name |
Endothelin A receptor (EDNRA)
|
|||||
| Synonyms |
HET-AR; Endothelin-1 receptor; Endothelin receptor type A; Endothelin receptor A; ETRA; ETA-R; ETA receptor; ETA; ET-A
Click to Show/Hide
|
|||||
| Gene Name |
EDNRA
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Cardiovascular disease [ICD-11: BA00-BE2Z] | |||||
| 2 | Pulmonary hypertension [ICD-11: BB01] | |||||
| 3 | Urinary system clinical sympton [ICD-11: MF8Y] | |||||
| Function |
Mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system. The rank order of binding affinities for ET-A is: ET1 > ET2 >> ET3. Receptor for endothelin-1.
Click to Show/Hide
|
|||||
| BioChemical Class |
GPCR rhodopsin
|
|||||
| UniProt ID | ||||||
| Sequence |
METLCLRASFWLALVGCVISDNPERYSTNLSNHVDDFTTFRGTELSFLVTTHQPTNLVLP
SNGSMHNYCPQQTKITSAFKYINTVISCTIFIVGMVGNATLLRIIYQNKCMRNGPNALIA SLALGDLIYVVIDLPINVFKLLAGRWPFDHNDFGVFLCKLFPFLQKSSVGITVLNLCALS VDRYRAVASWSRVQGIGIPLVTAIEIVSIWILSFILAIPEAIGFVMVPFEYRGEQHKTCM LNATSKFMEFYQDVKDWWLFGFYFCMPLVCTAIFYTLMTCEMLNRRNGSLRIALSEHLKQ RREVAKTVFCLVVIFALCWFPLHLSRILKKTVYNEMDKNRCELLSFLLLMDYIGINLATM NSCINPIALYFVSKKFKNCFQSCLCCCCYQSKSLMTSVPMNGTSIQWKNHDQNNHNTDRS SHKDSMN Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T34E5V | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 5 Approved Drugs | + | ||||
| 1 | Ambrisentan | Drug Info | Approved | Pulmonary arterial hypertension | [2], [3] | |
| 2 | Bosentan | Drug Info | Approved | Pulmonary arterial hypertension | [4], [5] | |
| 3 | LU302146 | Drug Info | Approved | Pulmonary hypertension | [6], [7] | |
| 4 | Macitentan | Drug Info | Approved | Cardiovascular disease | [8], [9], [10] | |
| 5 | Sparsentan | Drug Info | Approved | IgA nephropathy | [11] | |
| Clinical Trial Drug(s) | [+] 10 Clinical Trial Drugs | + | ||||
| 1 | Atrasentan | Drug Info | Phase 3 | Diabetic nephropathy | [12] | |
| 2 | Clazosentan | Drug Info | Phase 3 | Cerebral vasospasm | [13], [14] | |
| 3 | Darusentan | Drug Info | Phase 3 | Hypotension | [15], [16] | |
| 4 | PF-1228305 | Drug Info | Phase 3 | Pulmonary hypertension | [17] | |
| 5 | BQ-123 | Drug Info | Phase 2 | Pulmonary arterial hypertension | [18], [19] | |
| 6 | FR139317 | Drug Info | Phase 2 | Hypertension | [20], [21] | |
| 7 | PD-145065 | Drug Info | Phase 2 | Hypertension | [22] | |
| 8 | TBC-3711 | Drug Info | Phase 2 | Hypotension | [23] | |
| 9 | YM-598 | Drug Info | Phase 2 | Prostate cancer | [24] | |
| 10 | PMZ-2123 | Drug Info | Phase 1 | Cerebral oedema in diabetic ketoacidosis | [12] | |
| Discontinued Drug(s) | [+] 18 Discontinued Drugs | + | ||||
| 1 | Sitaxsentan | Drug Info | Withdrawn from market | Pulmonary arterial hypertension | [25], [3] | |
| 2 | TBC 11251 (TBC) | Drug Info | Withdrawn from market | Pulmonary arterial hypertension | [10], [26] | |
| 3 | Zibotentan | Drug Info | Discontinued in Phase 3 | Prostate cancer | [27], [28] | |
| 4 | 97-139 | Drug Info | Discontinued in Phase 2 | Cerebrovascular disease | [29] | |
| 5 | Avosentan | Drug Info | Discontinued in Phase 2 | Diabetic nephropathy | [30], [31] | |
| 6 | BMS-193884 | Drug Info | Discontinued in Phase 2 | Pulmonary arterial hypertension | [32] | |
| 7 | EDONENTAN HYDRATE | Drug Info | Discontinued in Phase 2 | Pulmonary arterial hypertension | [33] | |
| 8 | ENRASENTAN | Drug Info | Discontinued in Phase 2 | Pulmonary arterial hypertension | [34] | |
| 9 | J-104132 | Drug Info | Discontinued in Phase 2 | Pulmonary arterial hypertension | [35] | |
| 10 | SB-209670 | Drug Info | Discontinued in Phase 2 | Arrhythmia | [36], [37] | |
| 11 | A-216546 | Drug Info | Discontinued in Phase 1 | Solid tumour/cancer | [38] | |
| 12 | SB-234551 | Drug Info | Discontinued in Phase 1 | Nerve injury | [39], [40] | |
| 13 | ZD-1611 | Drug Info | Discontinued in Phase 1 | Hypotension | [41] | |
| 14 | 50-235 | Drug Info | Terminated | Hypertension | [42] | |
| 15 | BMS-182874 | Drug Info | Terminated | Hypertension | [43] | |
| 16 | BQ-518 | Drug Info | Terminated | Hypertension | [44] | |
| 17 | MX-6120 | Drug Info | Terminated | Heart disease | [45] | |
| 18 | PD-155080 | Drug Info | Terminated | Hypertension | [46] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Antagonist | [+] 31 Antagonist drugs | + | ||||
| 1 | Ambrisentan | Drug Info | [1] | |||
| 2 | LU302146 | Drug Info | [48] | |||
| 3 | Sparsentan | Drug Info | [49], [50] | |||
| 4 | Atrasentan | Drug Info | [51] | |||
| 5 | Clazosentan | Drug Info | [52] | |||
| 6 | Darusentan | Drug Info | [53] | |||
| 7 | PF-1228305 | Drug Info | [17] | |||
| 8 | BQ-123 | Drug Info | [54], [55] | |||
| 9 | FR139317 | Drug Info | [56] | |||
| 10 | TBC-3711 | Drug Info | [57] | |||
| 11 | YM-598 | Drug Info | [58] | |||
| 12 | PMZ-2123 | Drug Info | [12] | |||
| 13 | Sitaxsentan | Drug Info | [3] | |||
| 14 | TBC 11251 (TBC) | Drug Info | [59] | |||
| 15 | Zibotentan | Drug Info | [60] | |||
| 16 | 97-139 | Drug Info | [10], [61] | |||
| 17 | Avosentan | Drug Info | [62], [63] | |||
| 18 | EDONENTAN HYDRATE | Drug Info | [10], [65] | |||
| 19 | A-216546 | Drug Info | [10], [70] | |||
| 20 | SB-234551 | Drug Info | [10], [71] | |||
| 21 | ZD-1611 | Drug Info | [72] | |||
| 22 | BQ610 | Drug Info | [77] | |||
| 23 | MX-6120 | Drug Info | [78] | |||
| 24 | PD142893 | Drug Info | [77] | |||
| 25 | PD156707 | Drug Info | [81] | |||
| 26 | HJP-272 | Drug Info | [87] | |||
| 27 | LU302872 | Drug Info | [48] | |||
| 28 | PD164333 | Drug Info | [89] | |||
| 29 | [125I]PD151242 | Drug Info | [92] | |||
| 30 | [3H]BQ123 | Drug Info | [93] | |||
| 31 | [3H]S0139 | Drug Info | [87] | |||
| Modulator | [+] 11 Modulator drugs | + | ||||
| 1 | Bosentan | Drug Info | [47] | |||
| 2 | Macitentan | Drug Info | [9] | |||
| 3 | PD-145065 | Drug Info | [22] | |||
| 4 | BMS-193884 | Drug Info | [64] | |||
| 5 | ENRASENTAN | Drug Info | [66] | |||
| 6 | J-104132 | Drug Info | [67] | |||
| 7 | SB-209670 | Drug Info | [68], [69] | |||
| 8 | 50-235 | Drug Info | [74] | |||
| 9 | BQ-518 | Drug Info | [76] | |||
| 10 | PD-155080 | Drug Info | [79], [80] | |||
| 11 | BMS-187308 | Drug Info | [86] | |||
| Inhibitor | [+] 13 Inhibitor drugs | + | ||||
| 1 | ET-1 | Drug Info | [73] | |||
| 2 | BMS-182874 | Drug Info | [75] | |||
| 3 | 2-HYDROXY-3,5-DIIODOBENZOIC ACID | Drug Info | [82] | |||
| 4 | 4-(4-butylpiperidin-1-yl)-1-o-tolylbutan-1-one | Drug Info | [83] | |||
| 5 | Ac-bhg-F-N-Y-Y-W | Drug Info | [84] | |||
| 6 | Ac-w-F-F-N-Y-Y-W | Drug Info | [84] | |||
| 7 | Asterric acid | Drug Info | [85] | |||
| 8 | Bhg-F-N-Y-Y-W | Drug Info | [84] | |||
| 9 | Endothelin-2 | Drug Info | [73] | |||
| 10 | PD-163140 | Drug Info | [88] | |||
| 11 | Trp-Ile-Ile-Asp-Leu-Hisc(Cys-Ser-Val-Tyr-Phe-Cys) | Drug Info | [91] | |||
| 12 | Trp-Ile-Ile-Asp-Leu-Hisc(Cys-Val-Tyr-Phe-Cys) | Drug Info | [91] | |||
| 13 | W-F-F-N--Y-Y-W | Drug Info | [84] | |||
| Agonist | [+] 1 Agonist drugs | + | ||||
| 1 | sarafotoxin S6b | Drug Info | [90] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| cGMP-PKG signaling pathway | hsa04022 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| cAMP signaling pathway | hsa04024 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Neuroactive ligand-receptor interaction | hsa04080 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
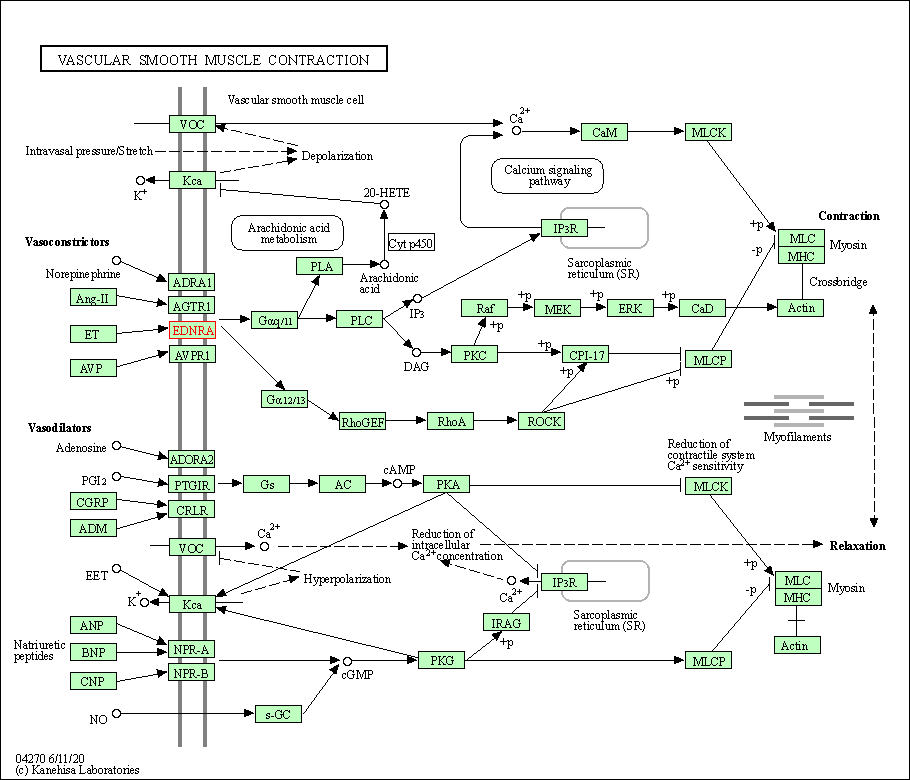
| Vascular smooth muscle contraction | hsa04270 | Affiliated Target |

|
| Class: Organismal Systems => Circulatory system | Pathway Hierarchy | ||
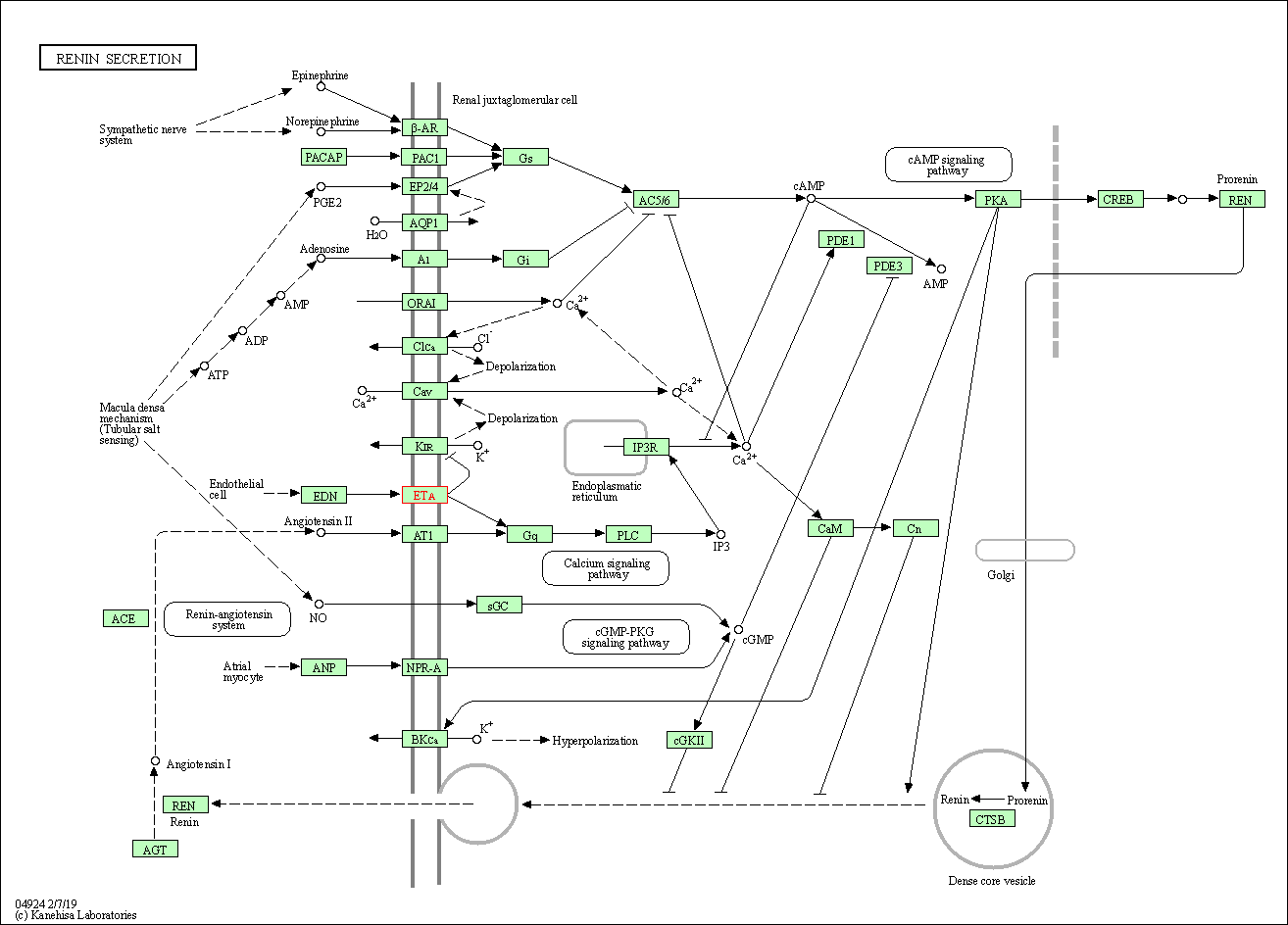
| Renin secretion | hsa04924 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 3 | Degree centrality | 3.22E-04 | Betweenness centrality | 3.11E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.98E-01 | Radiality | 1.34E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 6.67E+00 | Topological coefficient | 3.78E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 7 KEGG Pathways | + | ||||
| 1 | Calcium signaling pathway | |||||
| 2 | cGMP-PKG signaling pathway | |||||
| 3 | cAMP signaling pathway | |||||
| 4 | Neuroactive ligand-receptor interaction | |||||
| 5 | Vascular smooth muscle contraction | |||||
| 6 | Renin secretion | |||||
| 7 | Pathways in cancer | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | IL4 Signaling Pathway | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | Endothelin signaling pathway | |||||
| PID Pathway | [+] 2 PID Pathways | + | ||||
| 1 | Endothelins | |||||
| 2 | EGFR-dependent Endothelin signaling events | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Peptide ligand-binding receptors | |||||
| 2 | G alpha (q) signalling events | |||||
| WikiPathways | [+] 8 WikiPathways | + | ||||
| 1 | Prostaglandin Synthesis and Regulation | |||||
| 2 | GPCRs, Class A Rhodopsin-like | |||||
| 3 | Gastrin-CREB signalling pathway via PKC and MAPK | |||||
| 4 | Peptide GPCRs | |||||
| 5 | Endothelin Pathways | |||||
| 6 | GPCR ligand binding | |||||
| 7 | GPCR downstream signaling | |||||
| 8 | GPCRs, Other | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3951). | |||||
| REF 3 | Emerging treatments for pulmonary arterial hypertension. Expert Opin Emerg Drugs. 2006 Nov;11(4):609-19. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3494). | |||||
| REF 5 | Optimizing endothelin receptor antagonist use in the management of pulmonary arterial hypertension. Vasc Health Risk Manag. 2008;4(5):943-52. | |||||
| REF 6 | 2007 FDA drug approvals: a year of flux. Nat Rev Drug Discov. 2008 Feb;7(2):107-9. | |||||
| REF 7 | Ambrisentan, an endothelin receptor type A-selective endothelin receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2009 Aug;10(11):1847-58. | |||||
| REF 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7352). | |||||
| REF 9 | Radium 223 dichloride for prostate cancer treatment. Drug Des Devel Ther. 2017 Sep 6;11:2643-2651. | |||||
| REF 10 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 11 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 216403. | |||||
| REF 12 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 13 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 14 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 15 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3508). | |||||
| REF 16 | ClinicalTrials.gov (NCT00389779) DORADO-AC - Optimized Doses of Darusentan as Compared to an Active Control in Resistant Hypertension. U.S. National Institutes of Health. | |||||
| REF 17 | Pfizer. Product Development Pipeline. March 31 2009. | |||||
| REF 18 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 997). | |||||
| REF 19 | ClinicalTrials.gov (NCT00586820) Role of Endothelin in Microvascular Dysfunction Following PCI for NSTEMI. U.S. National Institutes of Health. | |||||
| REF 20 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 998). | |||||
| REF 21 | Acute Endothelin-Receptor Inhibition Does Not Attenuate Acetylcholine-Induced Coronary Vasoconstriction in Experimental Hypercholesterolemia. Arteriosclerosis, Thrombosis, and Vascular Biology, 1998.18: 108-113. | |||||
| REF 22 | Reversal of established responses to endothelin-1 in vivo and in vitro by the endothelin receptor antagonists, BQ-123 and PD 145065. Br J Pharmacol. 1994 May;112(1):207-13. | |||||
| REF 23 | ClinicalTrials.gov (NCT00272961) A Study Of Different Doses Of TBC3711 In Patients With Uncontrolled High Blood Pressure Already Taking Medications For High Blood Pressure.. U.S. National Institutes of Health. | |||||
| REF 24 | ClinicalTrials.gov (NCT00050297) YM598 in Patients With Rising PSA After Initial Therapy for Localized Prostate Cancer. U.S. National Institutes of Health. | |||||
| REF 25 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3950). | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007312) | |||||
| REF 27 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3539). | |||||
| REF 28 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009560) | |||||
| REF 29 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009311) | |||||
| REF 30 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8260). | |||||
| REF 31 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015393) | |||||
| REF 32 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007870) | |||||
| REF 33 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015788) | |||||
| REF 34 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004637) | |||||
| REF 35 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010566) | |||||
| REF 36 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3528). | |||||
| REF 37 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003996) | |||||
| REF 38 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010699) | |||||
| REF 39 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1000). | |||||
| REF 40 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009010) | |||||
| REF 41 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008908) | |||||
| REF 42 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003913) | |||||
| REF 43 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004811) | |||||
| REF 44 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800006825) | |||||
| REF 45 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018158) | |||||
| REF 46 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800005553) | |||||
| REF 47 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 48 | Influence of endothelin receptor antagonists on myocardial protein kinase C isoforms in uraemic cardiomyopathy. Clin Sci (Lond). 2002 Aug;103 Suppl 48:276S-279S. | |||||
| REF 49 | Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem. 2005 Oct 20;48(21):6523-43. | |||||
| REF 50 | DOI: 10.1038/hr.2009.135 | |||||
| REF 51 | Effects of a selective ET(A)-receptor antagonist, atrasentan (ABT-627), in murine models of allergic asthma: demonstration of mouse strain specificity. Clin Sci (Lond). 2002 Aug;103 Suppl 48:367S-370S. | |||||
| REF 52 | Effects of the selective endothelin A (ET(A)) receptor antagonist Clazosentan on cerebral perfusion and cerebral oxygenation following severe subar... Acta Neurochir (Wien). 2007;149(9):911-8; discussion 918. | |||||
| REF 53 | Endothelin ETA receptor blockade with darusentan increases sodium and potassium excretion in aging rats. J Cardiovasc Pharmacol. 2006 Mar;47(3):456-62. | |||||
| REF 54 | Endothelin in heart failure: a promising therapeutic target Heart. 1997 Feb;77(2):93-4. | |||||
| REF 55 | The therapeutic potential of endothelin-1 receptor antagonists and endothelin-converting enzyme inhibitors on the cardiovascular system. Expert Opin Investig Drugs. 2002 Nov;11(11):1537-52. | |||||
| REF 56 | Reduction of bFGF-induced smooth muscle cell proliferation and endothelin receptor mRNA expression by mevastatin and atorvastatin. Biochem Pharmacol. 2002 Aug 1;64(3):497-505. | |||||
| REF 57 | TBC3711, an ET(A) receptor antagonist, reduces neonatal hypoxia-induced pulmonary hypertension in piglets. Pediatr Res. 2001 Sep;50(3):374-83. | |||||
| REF 58 | Role of the endothelin axis and its antagonists in the treatment of cancer. Br J Pharmacol. 2011 May; 163(2): 220-233. | |||||
| REF 59 | Systemic administration of the endothelin-A receptor antagonist TBC 11251 attenuates cerebral vasospasm after experimental subarachnoid hemorrhage:... Neurosurgery. 1998 Dec;43(6):1409-17; discussion 1417-8. | |||||
| REF 60 | Clinical pipeline report, company report or official report of AstraZeneca (2009). | |||||
| REF 61 | S-0139 (Shionogi). Curr Opin Investig Drugs. 2002 Jul;3(7):1051-6. | |||||
| REF 62 | Agents in development for the treatment of diabetic nephropathy. Expert Opin Emerg Drugs. 2008 Sep;13(3):447-63. | |||||
| REF 63 | Influence of avosentan (SPP3OI) on the pharmacokinetics of a second generation oral contraceptive containing ethinylestradiol and levonorgestrel in healthy female volunteers. Int J Clin Pharmacol Ther. 2006 Dec;44(12):668-74. | |||||
| REF 64 | Vasodilator effects of the endothelin ETA receptor selective antagonist BMS-193884 in healthy men | |||||
| REF 65 | US patent application no. 9,062,094, Dipeptide-based prodrug linkers for aliphatic amine-containing drugs. | |||||
| REF 66 | Enrasentan, an antagonist of endothelin receptors. Cardiovasc Drug Rev. 2003 Spring;21(1):1-16. | |||||
| REF 67 | Pharmacological properties of J-104132 (L-753,037), a potent, orally active, mixed ETA/ETB endothelin receptor antagonist. J Pharmacol Exp Ther. 1999 Jun;289(3):1262-70. | |||||
| REF 68 | Effects of the endothelin receptor antagonist, SB 209670, on circulatory failure and organ injury in endotoxic shock in the anaesthetized rat. Br J Pharmacol. 1996 May;118(1):198-204. | |||||
| REF 69 | Nonpeptide endothelin receptor antagonists. III. Effect of SB 209670 and BQ123 on acute renal failure in anesthetized dogs. J Pharmacol Exp Ther. 1994 Nov;271(2):769-75. | |||||
| REF 70 | Pharmacology of A-216546: a highly selective antagonist for endothelin ET(A) receptor. Eur J Pharmacol. 1999 Feb 5;366(2-3):189-201. | |||||
| REF 71 | Effects of the novel selective endothelin ET(A) receptor antagonist, SB 234551, on the cardiovascular responses to endotoxaemia in conscious rats. Br J Pharmacol. 2001 Aug;133(8):1371-7. | |||||
| REF 72 | Pharmacological profile of ZD1611, a novel, orally active endothelin ETA receptor antagonist. J Pharmacol Exp Ther. 1999 Sep;290(3):1085-91. | |||||
| REF 73 | 5-OHKF and NorKA, depsipeptides from a Hawaiian collection of Bryopsis pennata: binding properties for NorKA to the human neuropeptide Y Y1 receptor. J Nat Prod. 2009 Dec;72(12):2172-6. | |||||
| REF 74 | Structure-activity relationships of an endothelin ETA receptor antagonist, 50-235, and its derivatives. Eur J Pharmacol. 1993 Oct 15;247(2):219-21. | |||||
| REF 75 | Biphenylsulfonamide endothelin receptor antagonists. 2. Discovery of 4'-oxazolyl biphenylsulfonamides as a new class of potent, highly selective ET... J Med Chem. 2000 Aug 10;43(16):3111-7. | |||||
| REF 76 | Structure-activity relationships of cyclic pentapeptide endothelin A receptor antagonists. J Med Chem. 1995 Oct 13;38(21):4309-24. | |||||
| REF 77 | Human optic nerve head astrocytes as a target for endothelin-1. Invest Ophthalmol Vis Sci. 2002 Aug;43(8):2704-13. | |||||
| REF 78 | Endothelin receptor antagonists in heart failure: current status and future directions. Drugs. 2004;64(10):1029-40. | |||||
| REF 79 | Potency of PD155080, an orally active ETA receptor antagonist, determined for human endothelin receptors. J Cardiovasc Pharmacol. 1995;26 Suppl 3:S362-4. | |||||
| REF 80 | Role of endothelin in hypertension of experimental chronic renal failure. Hypertension. 1997 Dec;30(6):1578-84. | |||||
| REF 81 | Discovery and development of an endothelin A receptor-selective antagonist PD 156707. Pharm Biotechnol. 1998;11:81-112. | |||||
| REF 82 | Allosteric inhibition of endothelin ETA receptors by 3, 5-dibromosalicylic acid. Mol Pharmacol. 2000 Dec;58(6):1461-9. | |||||
| REF 83 | Discovery of N-{1-[3-(3-oxo-2,3-dihydrobenzo[1,4]oxazin-4-yl)propyl]piperidin-4-yl}-2-phenylacetamide (Lu AE51090): an allosteric muscarinic M1 rec... J Med Chem. 2010 Sep 9;53(17):6386-97. | |||||
| REF 84 | Res-701-1, synthesis and a reevaluation of its effects on the endothelin receptors, Bioorg. Med. Chem. Lett. 5(6):621-626 (1995). | |||||
| REF 85 | New chlorinated diphenyl ethers from an Aspergillus species. J Nat Prod. 2002 Jan;65(1):7-10. | |||||
| REF 86 | Biphenylsulfonamide endothelin antagonists: structure-activity relationships of a series of mono- and disubstituted analogues and pharmacology of t... J Med Chem. 1998 Dec 17;41(26):5198-218. | |||||
| REF 87 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 219). | |||||
| REF 88 | gamma-Carbamate butenolide analogues as potent ETA selective endothelin receptor antagonists and prodrugs, Bioorg. Med. Chem. Lett. 7(3):297-302 (1997). | |||||
| REF 89 | Characterization of [125I]-PD164333, an ETA selective non-peptide radiolabelled antagonist, in normal and diseased human tissues. Br J Pharmacol. 1998 Jan;123(2):223-30. | |||||
| REF 90 | ETA receptor-mediated constrictor responses to endothelin peptides in human blood vessels in vitro. Br J Pharmacol. 1995 May;115(1):191-7. | |||||
| REF 91 | Structure-activity relationships in a series of monocyclic endothelin analogues, Bioorg. Med. Chem. Lett. 4(4):567-572 (1994). | |||||
| REF 92 | [125I]-PD151242: a selective radioligand for human ETA receptors. Br J Pharmacol. 1994 Jan;111(1):4-6. | |||||
| REF 93 | [3H]BQ-123, a highly specific and reversible radioligand for the endothelin ETA receptor subtype. Eur J Pharmacol. 1995 Feb 14;274(1-3):1-6. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

