Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0YS1U
|
|||
| Former ID |
DIB018038
|
|||
| Drug Name |
Sparsentan
|
|||
| Synonyms |
Sparsentan; RE-021; 254740-64-2; UNII-9242RO5URM; PS433540; PS-433540; CHEMBL539423; 9242RO5URM; BMS-346567; retrophin; Sparsentan [USAN]; compound 7 [PMID 15634011]; PS 33540; Sparsentan (RE-021); Sparsentan(PS433540); SCHEMBL535109; GTPL8448; BCP23969; BDBM50175523; SB16876; DB12548; CS-7947; DARA-a (Dual Acting Receptor Antagonist of angiotension and endothelin receptors); HY-17621; L023324; 4'-((2-butyl-4-oxo-1,3-diazaspiro[44]non-1-en-3-yl)methyl)-N-(4,5-dimethylisoxazol-3-yl)-2'-(ethoxymethyl)-[1,1'-biphenyl]-2-sulfonamide; RE-021
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | IgA nephropathy [ICD-11: MF8Y] | Approved | [1] | |
| Focal segmental glomerulosclerosis [ICD-11: MF8Y] | Phase 3 | [2] | ||
| Hypertension [ICD-11: BA00-BA04; ICD-9: 401] | Phase 2 | [3] | ||
| Myocardial infarction [ICD-11: BA41-BA43; ICD-9: 410] | Phase 2 | [4] | ||
| Company |
Travere Therapeutics
|
|||
| Structure |
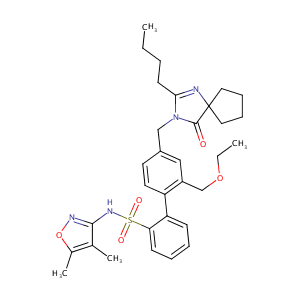 |
Download2D MOL |
||
| Formula |
C32H40N4O5S
|
|||
| Canonical SMILES |
CCCCC1=NC2(CCCC2)C(=O)N1CC3=CC(=C(C=C3)C4=CC=CC=C4S(=O)(=O)NC5=NOC(=C5C)C)COCC
|
|||
| InChI |
1S/C32H40N4O5S/c1-5-7-14-29-33-32(17-10-11-18-32)31(37)36(29)20-24-15-16-26(25(19-24)21-40-6-2)27-12-8-9-13-28(27)42(38,39)35-30-22(3)23(4)41-34-30/h8-9,12-13,15-16,19H,5-7,10-11,14,17-18,20-21H2,1-4H3,(H,34,35)
|
|||
| InChIKey |
WRFHGDPIDHPWIQ-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 254740-64-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 216403. | |||
| REF 2 | ClinicalTrials.gov (NCT03493685) Study of Sparsentan in Patients With Primary Focal Segmental Glomerulosclerosis (FSGS) (DUPLEX). U.S. National Institutes of Health. | |||
| REF 3 | ClinicalTrials.gov (NCT00635232) A Study To Evaluate The Dose-Related Efficacy and Safety of PS433540 in Subjects With Hypertension. U.S. National Institutes of Health. | |||
| REF 4 | Dual angiotensin II and endothelin A receptor antagonists: synthesis of 2'-substituted N-3-isoxazolyl biphenylsulfonamides with improved potency and pharmacokinetics. J Med Chem. 2005 Jan 13;48(1):171-9. | |||
| REF 5 | Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem. 2005 Oct 20;48(21):6523-43. | |||
| REF 6 | DOI: 10.1038/hr.2009.135 | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

