Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T74456
(Former ID: TTDS00267)
|
|||||
| Target Name |
Angiotensin II receptor type-1 (AGTR1)
|
|||||
| Synonyms |
Type-1 angiotensin II receptor; Angiotensin II type-1 receptor; Angiotensin II receptor 1; Angiotensin 1 receptor; AT2R1B; AT2R1; AT1BR; AT1AR; AT1; AGTR1B; AGTR1A
Click to Show/Hide
|
|||||
| Gene Name |
AGTR1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 4 Target-related Diseases | + | ||||
| 1 | Essential hypertension [ICD-11: BA00] | |||||
| 2 | Hypertension [ICD-11: BA00-BA04] | |||||
| 3 | Secondary hypertension [ICD-11: BA04] | |||||
| 4 | Urinary system clinical sympton [ICD-11: MF8Y] | |||||
| Function |
Mediates its action by association with G proteins that activate a phosphatidylinositol-calcium second messenger system. Receptor for angiotensin II.
Click to Show/Hide
|
|||||
| BioChemical Class |
GPCR rhodopsin
|
|||||
| UniProt ID | ||||||
| Sequence |
MILNSSTEDGIKRIQDDCPKAGRHNYIFVMIPTLYSIIFVVGIFGNSLVVIVIYFYMKLK
TVASVFLLNLALADLCFLLTLPLWAVYTAMEYRWPFGNYLCKIASASVSFNLYASVFLLT CLSIDRYLAIVHPMKSRLRRTMLVAKVTCIIIWLLAGLASLPAIIHRNVFFIENTNITVC AFHYESQNSTLPIGLGLTKNILGFLFPFLIILTSYTLIWKALKKAYEIQKNKPRNDDIFK IIMAIVLFFFFSWIPHQIFTFLDVLIQLGIIRDCRIADIVDTAMPITICIAYFNNCLNPL FYGFLGKKFKRYFLQLLKYIPPKAKSHSNLSTKMSTLSYRPSDNVSSSTKKPAPCFEVE Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T23RWU | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 16 Approved Drugs | + | ||||
| 1 | ANGIOTENSIN II | Drug Info | Approved | Increase blood pressure | [2] | |
| 2 | Azilsartan | Drug Info | Approved | Hypertension | [3], [4] | |
| 3 | Candesartan | Drug Info | Approved | Hypertension | [5], [6] | |
| 4 | Eprosartan | Drug Info | Approved | Hypertension | [6], [7] | |
| 5 | Forasartan | Drug Info | Approved | Hypertension | [8], [9] | |
| 6 | Irbesartan | Drug Info | Approved | Hypertension | [10], [11] | |
| 7 | Losartan | Drug Info | Approved | Hypertension | [11], [12] | |
| 8 | Micardis telmisartan | Drug Info | Approved | Hypertension | [13] | |
| 9 | Olmesartan medoxomil | Drug Info | Approved | High blood pressure | [14] | |
| 10 | Saprisartan | Drug Info | Approved | Hypertension | [15], [16] | |
| 11 | SARALASIN | Drug Info | Approved | Hypertension | [17], [18] | |
| 12 | Saralasin Acetate | Drug Info | Approved | Hypertension | [18] | |
| 13 | Sparsentan | Drug Info | Approved | IgA nephropathy | [19] | |
| 14 | Tasosartan | Drug Info | Approved | Hypertension | [6], [20] | |
| 15 | Telmisartan | Drug Info | Approved | Hypertension | [6], [21] | |
| 16 | Valsartan | Drug Info | Approved | Hypertension | [6], [22] | |
| Clinical Trial Drug(s) | [+] 6 Clinical Trial Drugs | + | ||||
| 1 | Pratosartan | Drug Info | Phase 3 | Hypertension | [23] | |
| 2 | S-474474 | Drug Info | Phase 3 | Metabolic syndrome x | [24] | |
| 3 | TAK-491 | Drug Info | Phase 3 | Hypertension | [25] | |
| 4 | CL-329167 | Drug Info | Phase 2 | Hypertension | [26] | |
| 5 | TRV027 | Drug Info | Phase 2 | Heart failure | [27] | |
| 6 | YM-358 | Drug Info | Phase 2 | Hypertension | [28] | |
| Discontinued Drug(s) | [+] 13 Discontinued Drugs | + | ||||
| 1 | KRH-594 | Drug Info | Discontinued in Phase 2 | Hypertension | [29] | |
| 2 | MILFASARTAN | Drug Info | Discontinued in Phase 2 | Hypertension | [30] | |
| 3 | RIPISARTAN | Drug Info | Discontinued in Phase 2 | Hypertension | [31] | |
| 4 | Zolasartan | Drug Info | Discontinued in Phase 2 | Hypotension | [32] | |
| 5 | TAK-591 | Drug Info | Discontinued in Phase 1 | Hypertension | [33] | |
| 6 | UR-7198 | Drug Info | Discontinued in Phase 1 | Hypertension | [34] | |
| 7 | A-81988 | Drug Info | Terminated | Hypertension | [35] | |
| 8 | CR-3834 | Drug Info | Terminated | Hypertension | [36] | |
| 9 | L-158809 | Drug Info | Terminated | Hypertension | [37] | |
| 10 | L-159689 | Drug Info | Terminated | Hypertension | [38] | |
| 11 | RS-66252 | Drug Info | Terminated | Alzheimer disease | [39] | |
| 12 | UP-275-22 | Drug Info | Terminated | Hypertension | [40] | |
| 13 | XR-510 | Drug Info | Terminated | Hypertension | [41] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Inhibitor | [+] 20 Inhibitor drugs | + | ||||
| 1 | ANGIOTENSIN II | Drug Info | [42] | |||
| 2 | SARALASIN | Drug Info | [56] | |||
| 3 | HELENALIN | Drug Info | [74] | |||
| 4 | L-159689 | Drug Info | [58] | |||
| 5 | 4-(2-Butyl-benzoimidazol-1-ylmethyl)-phenol | Drug Info | [80] | |||
| 6 | CV-11194 | Drug Info | [86] | |||
| 7 | GNF-PF-2307 | Drug Info | [74] | |||
| 8 | GNF-PF-2812 | Drug Info | [74] | |||
| 9 | GNF-PF-3832 | Drug Info | [74] | |||
| 10 | L-159093 | Drug Info | [58] | |||
| 11 | L-162313 | Drug Info | [89] | |||
| 12 | L-162782 | Drug Info | [89] | |||
| 13 | [Bpa1]AngII | Drug Info | [42] | |||
| 14 | [Sar1,Bpa2]AngII | Drug Info | [42] | |||
| 15 | [Sar1,Bpa3]AngII | Drug Info | [42] | |||
| 16 | [Sar1,Bpa8]AngII | Drug Info | [42] | |||
| 17 | [Sar1,Tdf2]AngII | Drug Info | [42] | |||
| 18 | [Sar1,Tdf3]AngII | Drug Info | [42] | |||
| 19 | [Sar1,Tdf8]AngII | Drug Info | [42] | |||
| 20 | [Tdf1]AngII | Drug Info | [42] | |||
| Modulator | [+] 7 Modulator drugs | + | ||||
| 1 | Azilsartan | Drug Info | [43] | |||
| 2 | Saralasin Acetate | Drug Info | [57] | |||
| 3 | TAK-491 | Drug Info | [43] | |||
| 4 | RS-66252 | Drug Info | [76] | |||
| 5 | UP-275-22 | Drug Info | [77] | |||
| 6 | XR-510 | Drug Info | [78] | |||
| 7 | CGP-49870 | Drug Info | [85] | |||
| Antagonist | [+] 35 Antagonist drugs | + | ||||
| 1 | Candesartan | Drug Info | [44], [45], [46] | |||
| 2 | Eprosartan | Drug Info | [47], [48] | |||
| 3 | Forasartan | Drug Info | [9], [49] | |||
| 4 | Irbesartan | Drug Info | [1], [50] | |||
| 5 | Losartan | Drug Info | [1], [51], [52], [53] | |||
| 6 | Micardis telmisartan | Drug Info | [54] | |||
| 7 | Olmesartan medoxomil | Drug Info | [55] | |||
| 8 | Saprisartan | Drug Info | [16] | |||
| 9 | Sparsentan | Drug Info | [58] | |||
| 10 | Tasosartan | Drug Info | [59] | |||
| 11 | Telmisartan | Drug Info | [60] | |||
| 12 | Valsartan | Drug Info | [1], [51], [61] | |||
| 13 | Pratosartan | Drug Info | [62] | |||
| 14 | CL-329167 | Drug Info | [63], [18] | |||
| 15 | TRV027 | Drug Info | [64] | |||
| 16 | YM-358 | Drug Info | [65], [18] | |||
| 17 | KRH-594 | Drug Info | [66] | |||
| 18 | MILFASARTAN | Drug Info | [67], [18] | |||
| 19 | RIPISARTAN | Drug Info | [68], [18] | |||
| 20 | TAK-591 | Drug Info | [70] | |||
| 21 | UR-7198 | Drug Info | [71] | |||
| 22 | A-81988 | Drug Info | [72] | |||
| 23 | CR-3834 | Drug Info | [73] | |||
| 24 | L-158809 | Drug Info | [75] | |||
| 25 | (E)-N-(3-iodoprop-2-enyl)-2beta-carbomethoxy-3beta-(4'-methylphenyl)n | Drug Info | [79] | |||
| 26 | 4-hydroxy-1-methyl-4-(4-methylphenyl)-3-piperidyl 4-methylphenyl ketone | Drug Info | [81] | |||
| 27 | 4-[(diphenylmethyl)amino]-2-phenylquinazoline | Drug Info | [82] | |||
| 28 | 5-butyl-methyl immidazole carboxylate 30 | Drug Info | [83] | |||
| 29 | 5-oxo-1-2-4-oxadiazol biphenyl | Drug Info | [84] | |||
| 30 | EXP3174 | Drug Info | [87], [88] | |||
| 31 | LY301875 | Drug Info | [91] | |||
| 32 | LY303336 | Drug Info | [91] | |||
| 33 | N,N`-bis-alkyl butylimmidazole 12b | Drug Info | [92] | |||
| 34 | [125I]EXP985 | Drug Info | [93] | |||
| 35 | [3H]GBR12935 | Drug Info | [94] | |||
| Blocker | [+] 1 Blocker drugs | + | ||||
| 1 | S-474474 | Drug Info | [24] | |||
| Agonist | [+] 3 Agonist drugs | + | ||||
| 1 | Zolasartan | Drug Info | [69] | |||
| 2 | angiotensin III | Drug Info | [77] | |||
| 3 | L-163,101 | Drug Info | [90] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: ZD-7155 | Ligand Info | |||||
| Structure Description | XFEL structure of human Angiotensin Receptor | PDB:4YAY | ||||
| Method | X-ray diffraction | Resolution | 2.90 Å | Mutation | Yes | [95] |
| PDB Sequence |
DLEDNWETLN
1011 DNLKVIEKAD1021 NAAQVKDALT1031 KMRAAALDAQ1041 KATPPKLEDK1051 SPDSPEMKDF 1061 RHGFDILVGQ1071 IDDALKLANE1081 GKVKEAQAAA1091 EQLKTTRNAY1101 IQKYLILNSS 16 DCPKAGRHNY26 IFVMIPTLYS36 IIFVVGIFGN46 SLVVIVIYFY56 MKLKTVASVF 66 LLNLALADLC76 FLLTLPLWAV86 YTAMEYRWPF96 GNYLCKIASA106 SVSFNLYASV 116 FLLTCLSIDR126 YLAIVHPMKS136 RLRRTMLVAK146 VTCIIIWLLA156 GLASLPAIIH 166 RNVFFIITVC180 AFHYETLPIG194 LGLTKNILGF204 LFPFLIILTS214 YTLIWKALKK 224 NDDIFKIIMA244 IVLFFFFSWI254 PHQIFTFLDV264 LIQLGIIRDC274 RIADIVDTAM 284 PITICIAYFN294 NCLNPLFYGF304 LGKKFKRYFL314 QLL
|
|||||
|
|
ILE31
4.353
TYR35
2.990
PHE77
3.956
LEU81
4.533
TRP84
3.484
TYR87
4.014
THR88
3.264
TYR92
4.774
ALA104
4.937
SER105
3.110
VAL108
3.679
|
|||||
| Ligand Name: Cholesterol | Ligand Info | |||||
| Structure Description | Structure of synthetic nanobody-stabilized angiotensin II type 1 receptor bound to TRV026 | PDB:6OS2 | ||||
| Method | X-ray diffraction | Resolution | 2.70 Å | Mutation | Yes | [96] |
| PDB Sequence |
IKRIQDDCPK
20 AGRHNYIFVM30 IPTLYSIIFV40 VGIFGNSLVV50 IVIYFYMKLK60 TVASVFLLNL 70 ALADLCFLLT80 LPLWAVYTAM90 EYRWPFGNYL100 CKIASASVSF110 NLYASVFLLT 120 CLSIDRYLAI130 VHPMKSRLRR140 TMLVAKVTCI150 IIWLLAGLAS160 LPAIIHRNVF 170 FIENTNITVC180 AFHYESQNST190 LPIGLGLTKN200 ILGFLFPFLI210 ILTSYTLIWK 220 ALKKAYDLED230 NWETLNDNLK240 VIEKADNAAQ250 VKDALTKMRA260 AALDAQKAHG 289 FDILVGQIDD299 ALKLANEGKV309 KEAQAAAEQL319 KKNKPRNDDI1238 FKIIMAIVLF 1248 FFFSWIPHQI1258 FTFLDVLIQL1268 GIIRDCRIAD1278 IVDTAMPITI1288 CIAYFNNCLN 1298 PLFYGFLGKK1308 FKRYFLQLLK1318
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
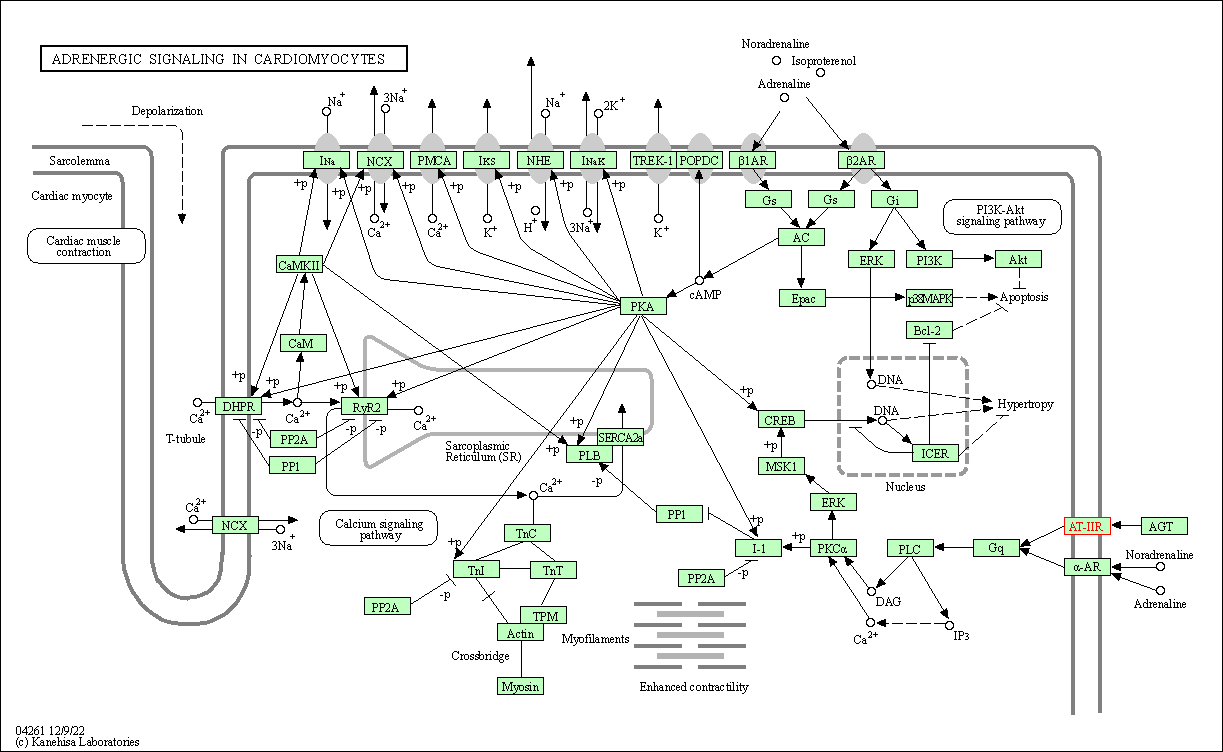
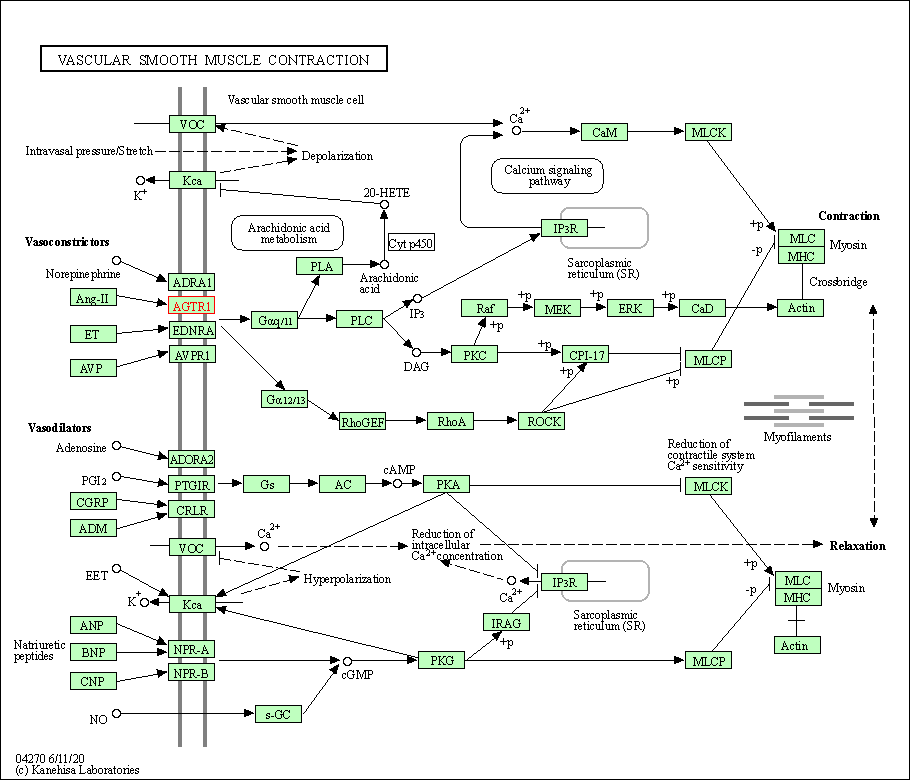
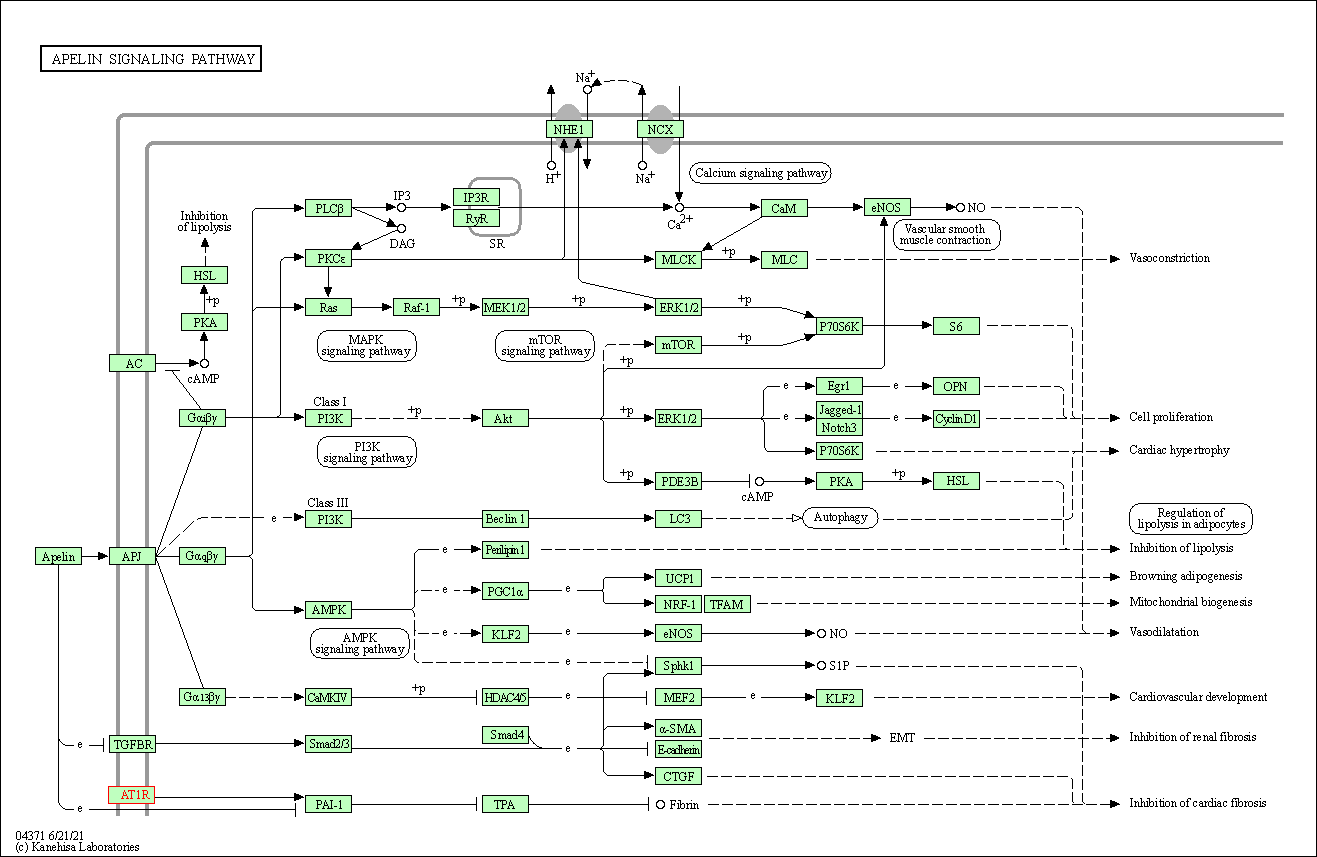
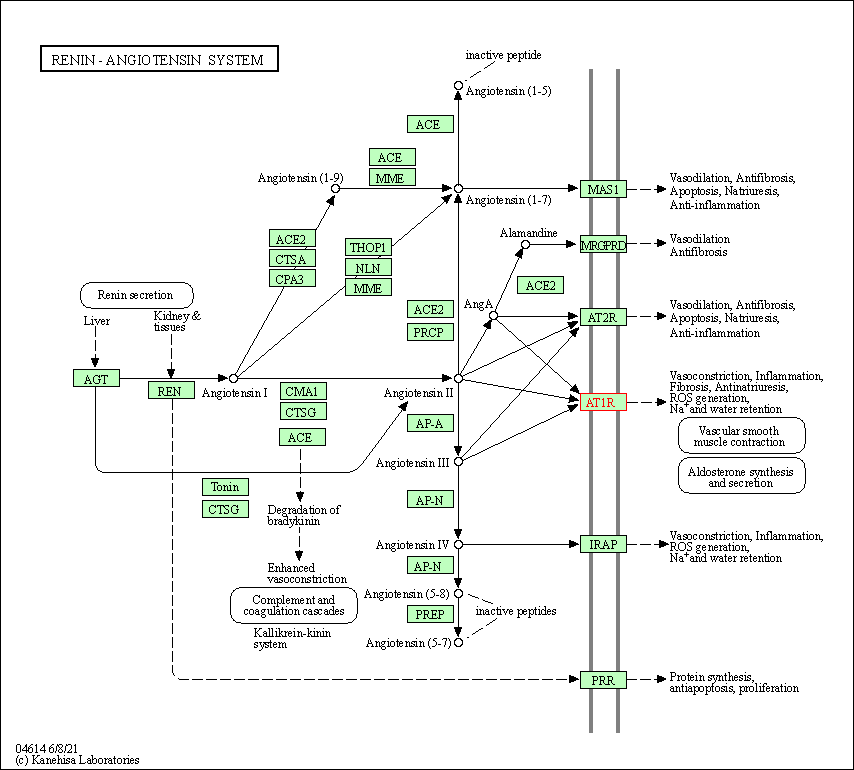
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Calcium signaling pathway | hsa04020 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| cGMP-PKG signaling pathway | hsa04022 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Phospholipase D signaling pathway | hsa04072 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Neuroactive ligand-receptor interaction | hsa04080 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Adrenergic signaling in cardiomyocytes | hsa04261 | Affiliated Target |

|
| Class: Organismal Systems => Circulatory system | Pathway Hierarchy | ||
| Vascular smooth muscle contraction | hsa04270 | Affiliated Target |

|
| Class: Organismal Systems => Circulatory system | Pathway Hierarchy | ||
| Apelin signaling pathway | hsa04371 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Renin-angiotensin system | hsa04614 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
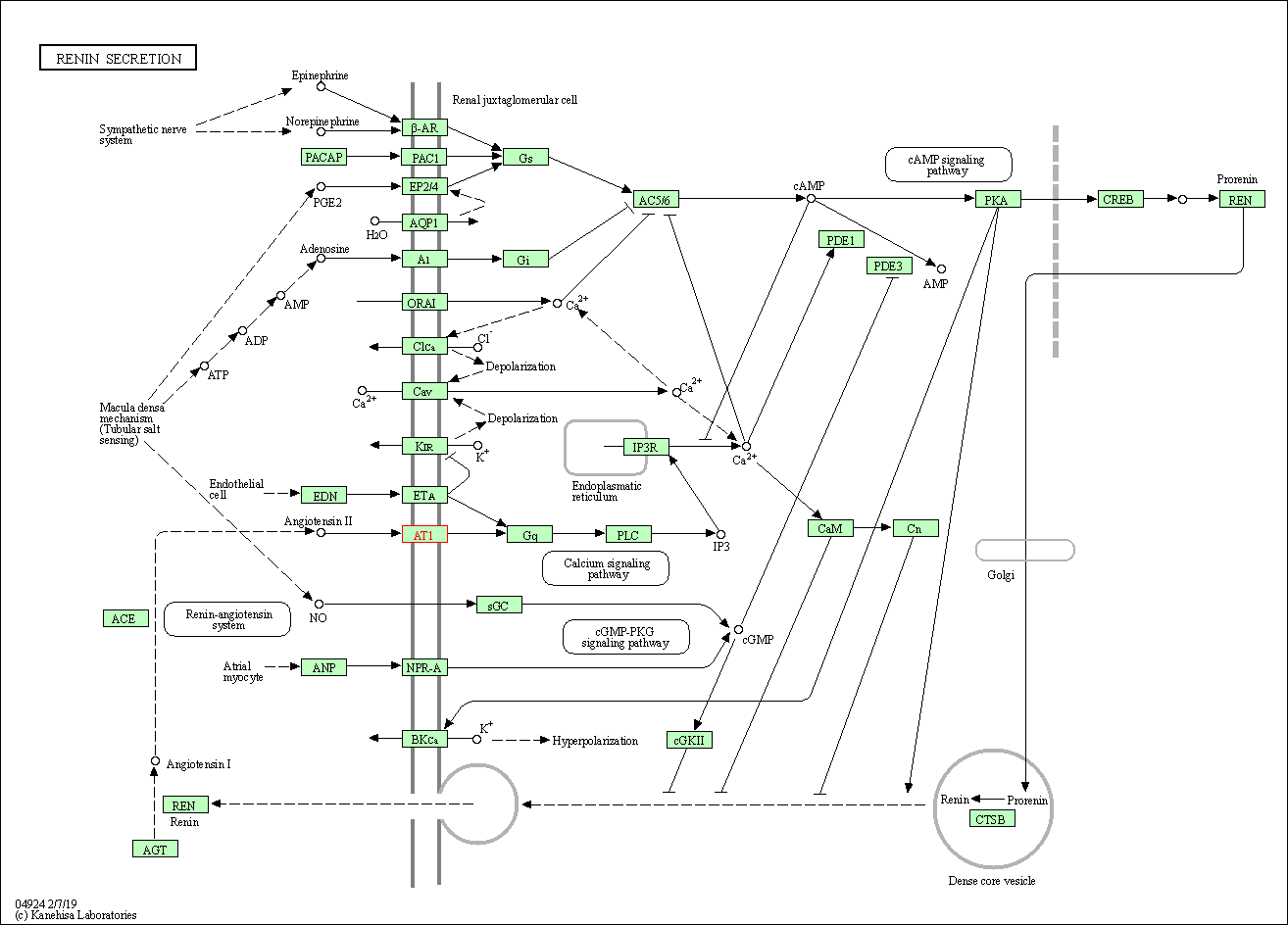
| Renin secretion | hsa04924 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
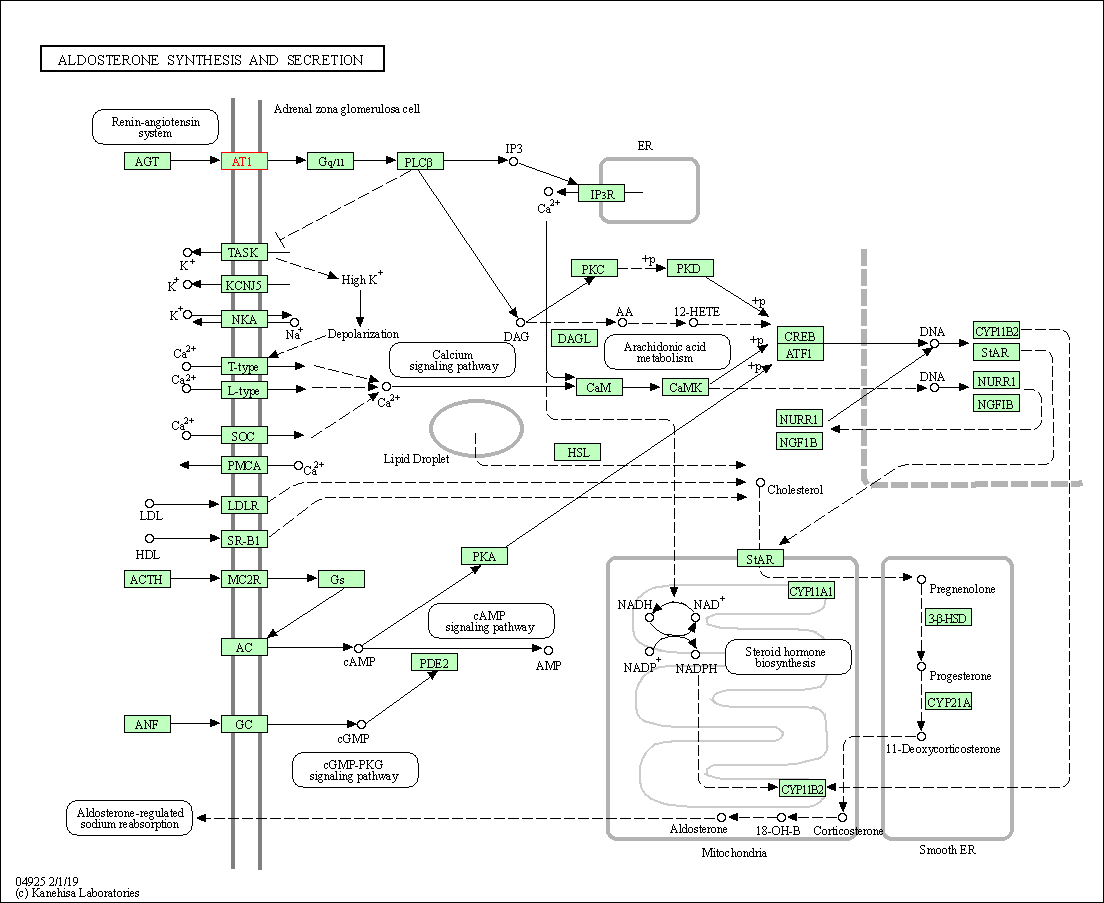
| Aldosterone synthesis and secretion | hsa04925 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
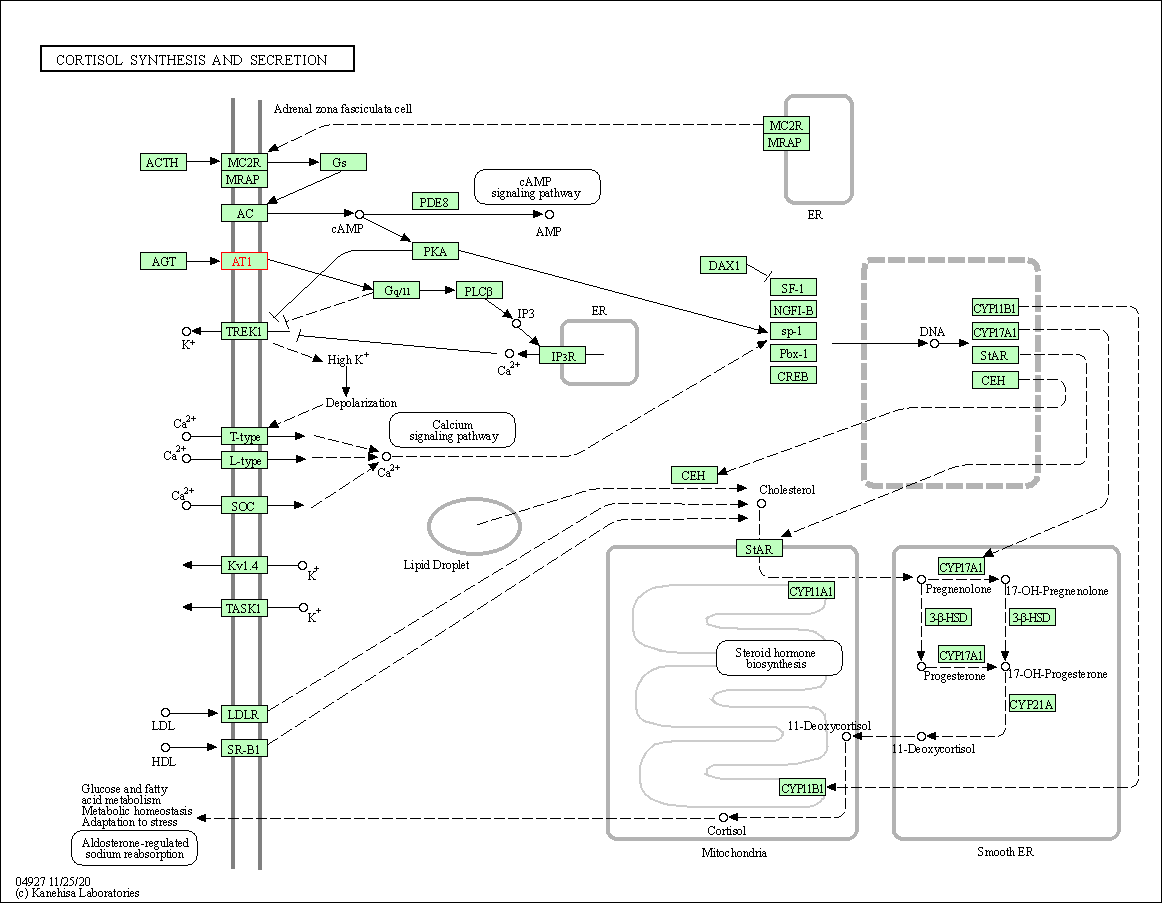
| Cortisol synthesis and secretion | hsa04927 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 10 | Degree centrality | 1.07E-03 | Betweenness centrality | 5.66E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.26E-01 | Radiality | 1.40E+01 | Clustering coefficient | 6.67E-02 |
| Neighborhood connectivity | 3.48E+01 | Topological coefficient | 1.25E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Radioligand binding assays: application of [(125)I]angiotensin II receptor binding. Methods Mol Biol. 2009;552:131-41. | |||||
| REF 2 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6901). | |||||
| REF 4 | Clinical pipeline report, company report or official report of Takeda. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 587). | |||||
| REF 6 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 588). | |||||
| REF 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6896). | |||||
| REF 9 | Non-peptide angiotensin II receptor antagonists: chemical feature based pharmacophore identification. J Med Chem. 2003 Feb 27;46(5):716-26. | |||||
| REF 10 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 589). | |||||
| REF 11 | New antiarrhythmic agents for atrial fibrillation and atrial flutter. Expert Opin Emerg Drugs. 2005 May;10(2):311-22. | |||||
| REF 12 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 590). | |||||
| REF 13 | Clinical pipeline report, company report or official report of Boehringer-Ingelheim Pharmaceuticals. | |||||
| REF 14 | Effect of angiotensin receptor blockade on endothelial function: focus on olmesartan medoxomil. Vasc Health Risk Manag. 2009;5(1):301-14. | |||||
| REF 15 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6899). | |||||
| REF 16 | Pharmacological properties of angiotensin II receptor antagonists. Can J Cardiol. 1999 Nov;15 Suppl F:26F-8F. | |||||
| REF 17 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 598). | |||||
| REF 18 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 19 | FDA Approved Drug Products from FDA Official Website. 2023. Application Number: 216403. | |||||
| REF 20 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6898). | |||||
| REF 21 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 592). | |||||
| REF 22 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3937). | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002623) | |||||
| REF 24 | Clinical pipeline report, company report or official report of Shionogi (2011). | |||||
| REF 25 | ClinicalTrials.gov (NCT02480764) TAK-491 (Azilsartan Medoxomil) Compared to Valsartan in Chinese Participants With Hypertension. | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004990) | |||||
| REF 27 | ClinicalTrials.gov (NCT01966601) A Study to Explore the Efficacy of TRV027 in Patients Hospitalized for Acute Decompensated Heart Failure. U.S. National Institutes of Health. | |||||
| REF 28 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002583) | |||||
| REF 29 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007913) | |||||
| REF 30 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002993) | |||||
| REF 31 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004530) | |||||
| REF 32 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001788) | |||||
| REF 33 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800028308) | |||||
| REF 34 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010597) | |||||
| REF 35 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002509) | |||||
| REF 36 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022642) | |||||
| REF 37 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001562) | |||||
| REF 38 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003495) | |||||
| REF 39 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007561) | |||||
| REF 40 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004864) | |||||
| REF 41 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004861) | |||||
| REF 42 | The amino-terminus of angiotensin II contacts several ectodomains of the angiotensin II receptor AT1. J Med Chem. 2010 Mar 11;53(5):2063-75. | |||||
| REF 43 | 2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4. | |||||
| REF 44 | Candesartan: widening indications for this angiotensin II receptor blocker Expert Opin Pharmacother. 2009 Aug;10(12):1995-2007. | |||||
| REF 45 | Angiotensin II type 1 receptor blockade: a novel therapeutic concept. Blood Press Suppl. 2000;1:9-13. | |||||
| REF 46 | Binding of the antagonist [3H]candesartan to angiotensin II AT1 receptor-transfected [correction of tranfected] Chinese hamster ovary cells. Eur J Pharmacol. 1999 Feb 19;367(2-3):413-22. | |||||
| REF 47 | Clinical profile of eprosartan: a different angiotensin II receptor blocker. Cardiovasc Hematol Agents Med Chem. 2008 Oct;6(4):253-7. | |||||
| REF 48 | Characterization of [3H]SK&F 108566 as a radioligand for angiotensin type-1 receptor. J Recept Res. 1993;13(5):849-61. | |||||
| REF 49 | Possible involvement of calcium-calmodulin pathways in the positive chronotropic response to angiotensin II on the canine cardiac sympathetic ganglia. Jpn J Pharmacol. 2001 Aug;86(4):381-9. | |||||
| REF 50 | Binding characteristics of [(3)H]-irbesartan to human recombinant angiotensin type 1 receptors. J Renin Angiotensin Aldosterone Syst. 2000 Jun;1(2):159-65. | |||||
| REF 51 | Knockouts model the 100 best-selling drugs--will they model the next 100 Nat Rev Drug Discov. 2003 Jan;2(1):38-51. | |||||
| REF 52 | Effect of episodic hypoxia on sympathetic activity and blood pressure. Respir Physiol. 2000 Feb;119(2-3):189-97. | |||||
| REF 53 | Characterization of [3H]losartan receptors in isolated rat glomeruli. Eur J Pharmacol. 1993 Oct 15;247(2):193-8. | |||||
| REF 54 | The angiotensin II receptor antagonist telmisartan reduces urinary albumin excretion in patients with isolated systolic hypertension: results of a randomized, double-blind, placebo-controlled trial. J Hypertens. 2005 Nov;23(11):2055-61. | |||||
| REF 55 | Mechanism of diastolic stiffening of the failing myocardium and its prevention by angiotensin receptor and calcium channel blockers. J Cardiovasc Pharmacol. 2009 Jul;54(1):47-56. | |||||
| REF 56 | Identification of a potent, selective, and orally active leukotriene a4 hydrolase inhibitor with anti-inflammatory activity. J Med Chem. 2008 Jul 24;51(14):4150-69. | |||||
| REF 57 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 58 | Designed multiple ligands. An emerging drug discovery paradigm. J Med Chem. 2005 Oct 20;48(21):6523-43. | |||||
| REF 59 | Tasosartan, enoltasosartan, and angiotensin II receptor blockade: the confounding role of protein binding. J Pharmacol Exp Ther. 2000 Nov;295(2):649-54. | |||||
| REF 60 | Deletion of angiotensin II type I receptor reduces hepatic steatosis. J Hepatol. 2009 Jun;50(6):1226-35. | |||||
| REF 61 | Interaction between the partially insurmountable antagonist valsartan and human recombinant angiotensin II type 1 receptors. Fundam Clin Pharmacol. 2000 Nov-Dec;14(6):577-85. | |||||
| REF 62 | Clinical efficacy of a new angiotensin II type 1 receptor blocker, pratosartan, in hypertensive patients. Hypertens Res. 2008 Feb;31(2):281-7. | |||||
| REF 63 | Distribution and function of cardiac angiotensin AT1- and AT2-receptor subtypes in hypertrophied rat hearts. Am J Physiol. 1994 Aug;267(2 Pt 2):H844-52. | |||||
| REF 64 | First clinical experience with TRV027: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2013 Sep;53(9):892-9. | |||||
| REF 65 | Effects of YM358, an angiotensin II type 1 (AT1) receptor antagonist, and enalapril on blood pressure and vasoconstriction in two renal hypertension models. Biol Pharm Bull. 2000 Feb;23(2):174-81. | |||||
| REF 66 | Binding of KRH-594, an antagonist of the angiotensin II type 1 receptor, to cloned human and rat angiotensin II receptors. Fundam Clin Pharmacol. 2002 Aug;16(4):317-23. | |||||
| REF 67 | Dual action molecules: bioassays of combined novel antioxidants and angiotensin II receptor antagonists. Eur J Pharmacol. 2012 Nov 15;695(1-3):96-103. | |||||
| REF 68 | In vitro pharmacological characterization of UP 269-6, a novel nonpeptide angiotensin II receptor antagonist. Fundam Clin Pharmacol. 1995;9(2):119-28. | |||||
| REF 69 | Azilsartan: a newly approved angiotensin II receptor blocker. Cardiol Rev. 2011 Nov-Dec;19(6):300-4. | |||||
| REF 70 | Clinical pipeline report, company report or official report of Takeda (2009). | |||||
| REF 71 | Pharmacologic profile of UR-7247, an orally active angiotensin II AT1 receptor antagonist, in healthy volunteers. p6. J Cardiovasc Pharmacol. 2000 Mar;35(3):383-9. | |||||
| REF 72 | [3H]A-81988, a potent, selective, competitive antagonist radioligand for angiotensin AT1 receptors. Eur J Pharmacol. 1994 Mar 15;267(1):49-54. | |||||
| REF 73 | Pharmacokinetic study of a new angiotensin-AT1 antagonist by HPLC. J Pharm Biomed Anal. 2008 Sep 29;48(2):422-7. | |||||
| REF 74 | In silico identification and biochemical evaluation of novel inhibitors of NRH:quinone oxidoreductase 2 (NQO2). Bioorg Med Chem Lett. 2010 Dec 15;20(24):7331-6. | |||||
| REF 75 | Characterization of the binding of [3H]L-158,809: a new potent and selective nonpeptide angiotensin II receptor (AT1) antagonist radioligand. Mol Pharmacol. 1992 Dec;42(6):1077-82. | |||||
| REF 76 | Virtual Screening in Drug Discovery, Juan Alvarez Brian Shoichet. Page(218). | |||||
| REF 77 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 34). | |||||
| REF 78 | Pharmacology of XR510, a potent orally active nonpeptide angiotensin II AT1 receptor antagonist with high affinity for the AT2 receptor subtype. J Cardiovasc Pharmacol. 1995 Sep;26(3):354-62. | |||||
| REF 79 | Pharmacological characterization of (E)-N-(3-iodoprop-2-enyl)-2beta-carbomethoxy-3beta-(4'-methylphenyl)n ortropane as a selective and potent inhibitor of the neuronal dopamine transporter. J Pharmacol Exp Ther. 1999 Nov;291(2):648-54. | |||||
| REF 80 | Discovery and development of aryl-fused imidazole-based angiotensin II antagonists, Bioorg. Med. Chem. Lett. 4(1):213-218 (1994). | |||||
| REF 81 | Discovery of a novel dopamine transporter inhibitor, 4-hydroxy-1-methyl-4-(4-methylphenyl)-3-piperidyl 4-methylphenyl ketone, as a potential cocaine antagonist through 3D-database pharmacophore searching. Molecular modeling, structure-activity relationships, and behavioral pharmacological studies. J Med Chem. 2000 Feb 10;43(3):351-60. | |||||
| REF 82 | Identification of a novel partial inhibitor of dopamine transporter among 4-substituted 2-phenylquinazolines. Bioorg Med Chem Lett. 2002 Aug 19;12(16):2225-8. | |||||
| REF 83 | The discovery of new potent non-peptide Angiotensin II AT1 receptor blockers: a concise synthesis, molecular docking studies and biological evaluation of N-substituted 5-butylimidazole derivatives. Eur J Med Chem. 2012 Sep;55:358-74. | |||||
| REF 84 | Synthesis and biological evaluation of novel potent angiotensin II receptor antagonists with anti-hypertension effect. Bioorg Med Chem. 2012 Apr 15;20(8):2747-61. | |||||
| REF 85 | Pharmaceutical compositions comprising NEP-inhibitors, inhibitors of the endogenous endothelin producing system and AT1 receptor antagonists | |||||
| REF 86 | Synthesis and angiotensin II receptor antagonistic activities of benzimidazole derivatives bearing acidic heterocycles as novel tetrazole bioisoste... J Med Chem. 1996 Dec 20;39(26):5228-35. | |||||
| REF 87 | Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993 Jun;45(2):205-51. | |||||
| REF 88 | Angiotensin II receptor antagonists: imidazoles and pyrroles bearing hydroxymethyl and carboxy substituents, Bioorg. Med. Chem. Lett. 4(1):177-182 (1994). | |||||
| REF 89 | Selective angiotensin II AT2 receptor agonists: Benzamide structure-activity relationships. Bioorg Med Chem. 2008 Jul 15;16(14):6841-9. | |||||
| REF 90 | Structural model of antagonist and agonist binding to the angiotensin II, AT1 subtype, G protein coupled receptor. Chem Biol. 1994 Dec;1(4):211-21. | |||||
| REF 91 | Inhibition of angiotensin II-induced inositol phosphate production by triacid nonpeptide antagonists in CHO cells expressing human AT1 receptors. Pharm Res. 2000 Dec;17(12):1482-8. | |||||
| REF 92 | Rational design, efficient syntheses and biological evaluation of N,N'-symmetrically bis-substituted butylimidazole analogs as a new class of potent Angiotensin II receptor blockers. Eur J Med Chem. 2013 Apr;62:352-70. | |||||
| REF 93 | [125I]EXP985: a highly potent and specific nonpeptide radioligand antagonist for the AT1 angiotensin receptor. Biochem Biophys Res Commun. 1992 Nov 16;188(3):1030-9. | |||||
| REF 94 | Specific binding of 1-[2-(diphenylmethoxy)ethyl]-4-(3-phenyl propyl) piperazine (GBR-12935), an inhibitor of the dopamine transporter, to human CYP2D6. Biochem Pharmacol. 1997 Jun 15;53(12):1937-9. | |||||
| REF 95 | Structure of the Angiotensin receptor revealed by serial femtosecond crystallography. Cell. 2015 May 7;161(4):833-44. | |||||
| REF 96 | Angiotensin and biased analogs induce structurally distinct active conformations within a GPCR. Science. 2020 Feb 21;367(6480):888-892. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

