Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0Z6UC
|
|||
| Former ID |
DAP000219
|
|||
| Drug Name |
Sumatriptan
|
|||
| Synonyms |
Sumatran; Sumatriptanum; Sumax; GR 43175; GR 43175X; NP101; GR-43175; Imigran (TN); Imitrex (TN); KS-1116; Sumatriptanum [INN-Latin]; Sumatriptan (JAN/USP/INN); (3-[2-(Dimethylamino)ethyl]-1H-indol-5-yl)-N-methylmethanesulfonamide; 1-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-N-methyl-methanesulfonamide; 1-[3-(2-dimethylaminoethyl)-1H-indol-5-yl]-N-methylmethanesulfonamide; 1-{3-[2-(dimethylamino)ethyl]-1H-indol-5-yl}-N-methylmethanesulfonamide; 3-(2-(Dimethylamino)ethyl)-N-methyl-1H-indole-5-methanesulfonamide; 3-[2-(Dimethylamino)ethyl]-N-methyl-1H-indole-5-methanesulfonamide; 3-[2-(Dimethylamino)ethyl]-N-methylindole-5-methanesulfonamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Migraine [ICD-11: 8A80; ICD-10: G43, G43.9; ICD-9: 346] | Approved | [1], [2] | |
| Therapeutic Class |
Vasoconstrictor Agents
|
|||
| Company |
GlaxoSmithKline
|
|||
| Structure |
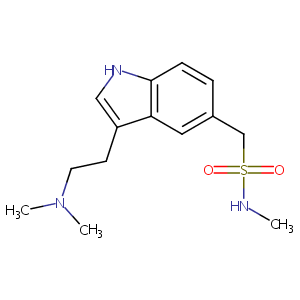 |
Download2D MOL |
||
| Formula |
C14H21N3O2S
|
|||
| Canonical SMILES |
CNS(=O)(=O)CC1=CC2=C(C=C1)NC=C2CCN(C)C
|
|||
| InChI |
1S/C14H21N3O2S/c1-15-20(18,19)10-11-4-5-14-13(8-11)12(9-16-14)6-7-17(2)3/h4-5,8-9,15-16H,6-7,10H2,1-3H3
|
|||
| InChIKey |
KQKPFRSPSRPDEB-UHFFFAOYSA-N
|
|||
| CAS Number |
CAS 103628-46-2
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
9527, 5634131, 7847517, 7980713, 8153293, 10502764, 14718471, 14825017, 25819952, 26612933, 26749855, 29224410, 46506520, 48334598, 48416587, 49679316, 49984205, 50107736, 50422744, 53790264, 56322605, 57322735, 81040912, 85209322, 85789242, 93166406, 103188807, 103919260, 104309008, 119526324, 124799677, 125336887, 126525330, 126667002, 128419875, 134337534, 135018088, 135651095, 137002439, 142467611, 144205081, 152034343, 160964014, 162182413, 164814822, 165699400, 170465377, 172866507, 174006309, 175265694
|
|||
| ChEBI ID |
CHEBI:10650
|
|||
| ADReCS Drug ID | BADD_D02098 ; BADD_D02099 | |||
| SuperDrug ATC ID |
N02CC01
|
|||
| SuperDrug CAS ID |
cas=103628462
|
|||
| Interaction between the Drug and Microbe | Top | |||
|---|---|---|---|---|
| The Metabolism of Drug Affected by Studied Microbe(s) | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Bacteroidales | ||||
|
Studied Microbe: Bacteroides fragilis HMW 610
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Sumatriptan succinate can be metabolized by Bacteroides fragilis HMW 610 (log2FC = -0.502; p = 0.016). | |||
|
Studied Microbe: Bacteroides fragilis str. DS-208
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Sumatriptan succinate can be metabolized by Bacteroides fragilis str. DS-208 (log2FC = -0.623; p = 0.006). | |||
|
Studied Microbe: Bacteroides thetaiotaomicron VPI-5482
Show/Hide Hierarchy
|
[3] | |||
| Hierarchy | ||||
| Experimental Method | High-throughput screening | |||
| Description | Sumatriptan succinate can be metabolized by Bacteroides thetaiotaomicron VPI-5482 (log2FC = -0.413; p = 0.025). | |||
| The Abundace of Studied Microbe(s) Regulated by Drug | ||||
| The Order in the Taxonomic Hierarchy of the following Microbe(s): Verrucomicrobiales | ||||
|
Studied Microbe: Akkermansia muciniphila
Show/Hide Hierarchy
|
[4] | |||
| Hierarchy | ||||
| Abundance Change | Decrease | |||
| Experiment Method | High-throughput screening | |||
| Description | The abundance of Akkermansia muciniphila was decreased by Sumatriptan succinate (adjusted p-values: 8.31E-03). | |||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 1D receptor (HTR1D) | Target Info | Agonist | [5], [6], [7], [8], [9] |
| KEGG Pathway | cAMP signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Serotonergic synapse | ||||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| 5HT1 type receptor mediated signaling pathway | ||||
| Reactome | Serotonin receptors | |||
| G alpha (i) signalling events | ||||
| WikiPathways | Serotonin HTR1 Group and FOS Pathway | |||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 54). | |||
| REF 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 3 | Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019 Jun;570(7762):462-467. | |||
| REF 4 | Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018 Mar 29;555(7698):623-628. | |||
| REF 5 | Irritable bowel syndrome: new agents targeting serotonin receptor subtypes. Drugs. 2001;61(3):317-32. | |||
| REF 6 | 5-Hydroxytryptamine(1F) receptors do not participate in vasoconstriction: lack of vasoconstriction to LY344864, a selective serotonin(1F) receptor agonist in rabbit saphenous vein. J Pharmacol Exp Ther. 1999 Sep;290(3):935-9. | |||
| REF 7 | 5-Hydroxytryptamine receptor agonists for the abortive treatment of vascular headaches block mast cell, endothelial and platelet activation within the rat dura mater after trigeminal stimulation. Brain Res. 1992 Jun 26;583(1-2):137-49. | |||
| REF 8 | Novel approaches to the treatment of nausea and vomiting. Dig Dis. 1999;17(3):125-32. | |||
| REF 9 | Serotonin in migraine: theories, animal models and emerging therapies. Prog Drug Res. 1998;51:219-44. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

