Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D34RXC
|
|||
| Drug Name |
VIP-152
|
|||
| Synonyms |
Enitociclib; 1255AT22ZJ; UNII-1255AT22ZJ; VIP152; CHEMBL4762845; VIP-152; BAY-1251152; 1610408-97-3; 2-Pyridinamine, 5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-(4-(((S(S))-S-methylsulfonimidoyl)methyl)-2-pyridinyl)-; 2-Pyridinamine, 5-fluoro-4-(4-fluoro-2-methoxyphenyl)-N-[4-[[[S(S)]-S-methylsulfonimidoyl]methyl]-2-pyridinyl]-; ENITOCICLIB [INN]; ENITOCICLIB [USAN]; GTPL11686; GLXC-26844; BDBM50556034; BAY 1251152; csc(S)-[(2-{[5-fluoro-4-(4-fluoro-2-methoxyphenyl)pyridin-2-yl]amino}pyridin-4-yl)methyl](imino)(methyl)-l6-sulfanone
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Chronic lymphocytic leukaemia [ICD-11: 2A82.0; ICD-10: C83.0, C91.1] | Phase 1 | [1] | |
| Non-hodgkin lymphoma [ICD-11: 2B33.5; ICD-10: C82-C85; ICD-9: 200, 202] | Phase 1 | [1] | ||
| Company |
Vincerx Pharma Palo Alto, CA
|
|||
| Structure |
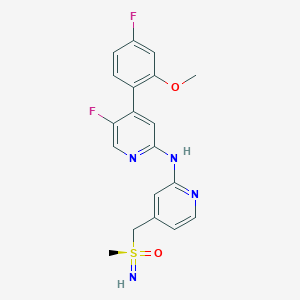 |
Download2D MOL |
||
| Formula |
C19H18F2N4O2S
|
|||
| Canonical SMILES |
COC1=C(C=CC(=C1)F)C2=CC(=NC=C2F)NC3=NC=CC(=C3)CS(=N)(=O)C
|
|||
| InChI |
InChI=1S/C19H18F2N4O2S/c1-27-17-8-13(20)3-4-14(17)15-9-19(24-10-16(15)21)25-18-7-12(5-6-23-18)11-28(2,22)26/h3-10,22H,11H2,1-2H3,(H,23,24,25)/t28-/m0/s1
|
|||
| InChIKey |
YZCUMZWULWOUMD-NDEPHWFRSA-N
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Cyclin-dependent kinase 9 (CDK9) | Target Info | Inhibitor | [2] |
| KEGG Pathway | Transcriptional misregulation in cancer | |||
| NetPath Pathway | EGFR1 Signaling Pathway | |||
| Reactome | SMAD2/SMAD3:SMAD4 heterotrimer regulates transcription | |||
| WikiPathways | Cardiac Hypertrophic Response | |||
| Transcriptional activity of SMAD2/SMAD3:SMAD4 heterotrimer | ||||
| Host Interactions of HIV factors | ||||
| HIV Life Cycle | ||||
| IL-9 Signaling Pathway | ||||
| RNA Polymerase II Transcription | ||||
| MicroRNAs in cardiomyocyte hypertrophy | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT04978779) An Open-label, Multicenter Phase 1/1b Study to Characterize Safety, Tolerability, Preliminary Antitumor Activity, Pharmacokinetics, and Pharmacodynamics of VIP152 Monotherapy or Combination Therapy in Subjects With High-risk Chronic Lymphocytic Leukemia or Richter Syndrome. U.S.National Institutes of Health. | |||
| REF 2 | VIP152 is a selective CDK9 inhibitor with pre-clinical in vitro and in vivo efficacy in chronic lymphocytic leukemia. Leukemia. 2023 Feb;37(2):326-338. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

