Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D5E2KR
|
|||
| Drug Name |
ANG-3777
|
|||
| Synonyms |
Terevalefim; Refanalin; Terevalefim [INN]; ANG-3777; 1070881-42-3; Terevalefim [USAN]; SNV-003; 500128-99-4; GG91UXK2M5; Terevalefim [USAN:INN]; UNII-GG91UXK2M5; 5-((E)-2-Thiophen-2-yl-vinyl)-lh-pyrazole; 5-[(E)-2-thiophen-2-ylethenyl]-1H-pyrazole; (e)-5-(2-(thiophen-2-yl)vinyl)-1h-pyrazole; WHO 11458; 3-[2-(2-thienyl)ethenyl]-1H-Pyrazole; 1H-Pyrazole, 3-((1E)-2-(2-thienyl)ethenyl)-; BB3; TEREVALEFIM [WHO-DD]; BB-3; SCHEMBL1225335; CHEMBL4650326; SCHEMBL10149623; GTPL11997; ANG3777; GLXC-26914; AKOS040755926; MS-22944; HY-137455; 5-[2-(thiophen-2-yl)ethenyl]-1H-pyrazole; CS-0138662; E80357; (E)-3-(2-(thiophen-2-yl)vinyl)-1H-pyrazole; EN300-7830322; 5-[(E)-2-(thiophen-2-yl)ethenyl]-1H-pyrazole; EN300-18541705
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Delayed graft function [ICD-11: 4B24.0; ICD-10: T86; ICD-9: 996] | Phase 3 | [1] | |
| Company |
Angion Biomedica
|
|||
| Structure |
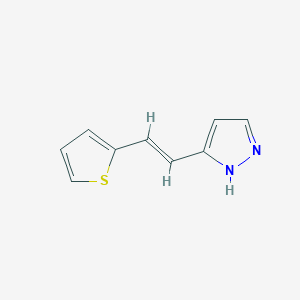 |
Download2D MOL
|
||
| Formula |
C9H8N2S
|
|||
| Canonical SMILES |
C1=CSC(=C1)C=CC2=CC=NN2
|
|||
| InChI |
InChI=1S/C9H8N2S/c1-2-9(12-7-1)4-3-8-5-6-10-11-8/h1-7H,(H,10,11)/b4-3+
|
|||
| InChIKey |
FOHWAQGURRYJFK-ONEGZZNKSA-N
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT02474667) A Multicenter, Prospective, Double-Blind, Randomized, Placebo-Controlled, Phase 3 Study of ANG-3777 (Formerly BB3) to Improve Graft Function and Reduce the Severity of Kidney Dysfunction or Delayed Graft Function Following Kidney Transplantation in Recipients of a Deceased Donor Kidney. U.S.National Institutes of Health. | |||
| REF 2 | Hepatocyte Growth Factor Mimetic ANG-3777 for Cardiac Surgery-Associated Acute Kidney Injury. Kidney Int Rep. 2020 Sep 25;5(12):2325-2332. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

