Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D5XVM6
|
|||
| Drug Name |
Zabedosertib
|
|||
| Synonyms |
Zabedosertib; 1931994-81-8; N-[6-(1-hydroxy-1-methyl-ethyl)-2-(2-methylsulfonylethyl)indazol-5-yl]-6-(trifluoromethyl)pyridine-2-carboxamide; Zabedosertib [INN]; BAY-1834845; N1GRK350ZM; BAY1834845; BAY 1834845; 2-Pyridinecarboxamide, N-(6-(1-hydroxy-1-methylethyl)-2-(2-(methylsulfonyl)ethyl)-2H-indazol-5-yl)-6-(trifluoromethyl)-; N-(6-(1-Hydroxy-1-methylethyl)-2-(2-(methylsulfonyl)ethyl)-2H-indazol-5-yl)-6-(trifluoromethyl)-2-pyridinecarboxamide; N-(6-(2-Hydroxypropan-2-yl)-2-(2-(methanesulfonyl)ethyl)- 2H-indazol-5-yl)-6-(trifluoromethyl)pyridine-2- carboxamide; N-(6-(2-Hydroxypropan-2-yl)-2-(2-(methylsulfonyl)ethyl)-2H-indazol-5-yl)-6-(trifluoromethyl)picolinamide; N-[6-(2-hydroxypropan-2-yl)-2-(2-methylsulfonylethyl)indazol-5-yl]-6-(trifluoromethyl)pyridine-2-carboxamide; UNII-N1GRK350ZM; SCHEMBL17785221; GTPL11415; OQAMEEFUUFJZRS-UHFFFAOYSA-N; BDBM395297; EX-A5143; US10308634, Example 12; MFCD32900903; AKOS040755617; SB74225; MS-28697; SY323200; HY-139374; CS-0198831; D96922; N-[6-(2-Hydroxy-2-propyl)-2-[2-(methylsulfonyl)ethyl]-2H-indazol-5-yl]-6-(trifluoromethyl)pyridine-2-carboxamide; N-{6-(2-Hydroxypropan-2-yl)-2-[2-(methylsulphonyl)ethyl]-2H-indazol-5-yl}-6-(trifluoromethyl)pyridine-2-carboxamide
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Atopic dermatitis [ICD-11: EA80; ICD-10: L20] | Phase 2 | [1] | |
| Company |
Bayer
|
|||
| Structure |
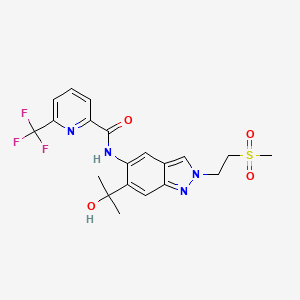 |
Download2D MOL |
||
| Formula |
C20H21F3N4O4S
|
|||
| Canonical SMILES |
CC(C)(C1=CC2=NN(C=C2C=C1NC(=O)C3=NC(=CC=C3)C(F)(F)F)CCS(=O)(=O)C)O
|
|||
| InChI |
InChI=1S/C20H21F3N4O4S/c1-19(2,29)13-10-15-12(11-27(26-15)7-8-32(3,30)31)9-16(13)25-18(28)14-5-4-6-17(24-14)20(21,22)23/h4-6,9-11,29H,7-8H2,1-3H3,(H,25,28)
|
|||
| InChIKey |
OQAMEEFUUFJZRS-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05656911) A Randomized, Placebo-controlled, Double-blind, Parallel-group, Multicenter Phase 2a Study to Investigate Efficacy and Safety of Zabedosertib (BAY 1834845) for the Treatment of Adult Patients With Moderate-to-severe Atopic Dermatitis. U.S.National Institutes of Health. | |||
| REF 2 | Oral IRAK4 inhibitor BAY-1834845 prevents acute respiratory distress syndrome. Biomed Pharmacother. 2022 Sep;153:113459. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

