Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D63WMQ
|
|||
| Drug Name |
Ingrezza
|
|||
| Synonyms |
Valbenazine; 1025504-45-3; UNII-54K37P50KH; Valbenazine free base; NBI 98854; 54K37P50KH; 1025504-45-3 (free base); (2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl L-valinate; L-Valine, (2R,3R,11bR)-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo[a]quinolizin-2-yl ester; Valbenazine [USAN:INN]; L-Valine, (2R,3R,11bR)-1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo(a)quinolizin-2-yl ester; ValbenazineNBI-98854; Valbenazine (USAN/INN); GTPL8694; CHEMBL2364639; SCHEMBL15932979; EX-A2002; MFCD28963976; ZINC43195697; CS-5908; DB11915; SB17456; NCGC00522306-02; [(2R,3R,11bR)-9,10-dimethoxy-3-(2-methylpropyl)-2,3,4,6,7,11b-hexahydro-1H-benzo[a]quinolizin-2-yl] (2S)-2-amino-3-methylbutanoate; AC-30929; AS-35294; HY-16771; D10675; NBI-98854;NBI98854;NBI 98854; Q27089118; (2R,3R,11bR)-9,10-Dimethoxy-3-(2-methylpropyl)-1,3,4,6,7,11b-hexahydro-2H- benzo(a)quinolizin-2-yl L-valinate; (S)-2-amino-3-methyl-butyric acid (2R,3R,11bR)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2H-pyrido[2,1-a]isoquinolin-2-yl ester; [(2R,3R,11bR)-9,10-dimethoxy-3-(2-methylpropyl)-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinolin-2-yl] (2S)-2-amino-3-methylbutanoate; Valine 1,3,4,6,7,11b-hexahydro-9,10-dimethoxy-3-(2-methylpropyl)-2H-benzo(a)quinolizin-2-yl ester
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Tardive dyskinesia [ICD-11: 8A02.10] | Phase 4 | [1] | |
| Tourette syndrome [ICD-11: 8A05.00; ICD-10: F95.2] | Phase 2 | [2] | ||
| Company |
Neurocrine Biosciences
|
|||
| Structure |
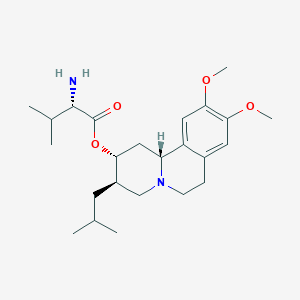 |
Download2D MOL |
||
| Formula |
C24H38N2O4
|
|||
| Canonical SMILES |
CC(C)CC1CN2CCC3=CC(=C(C=C3C2CC1OC(=O)C(C(C)C)N)OC)OC
|
|||
| InChI |
1S/C24H38N2O4/c1-14(2)9-17-13-26-8-7-16-10-21(28-5)22(29-6)11-18(16)19(26)12-20(17)30-24(27)23(25)15(3)4/h10-11,14-15,17,19-20,23H,7-9,12-13,25H2,1-6H3/t17-,19-,20-,23+/m1/s1
|
|||
| InChIKey |
GEJDGVNQKABXKG-CFKGEZKQSA-N
|
|||
| CAS Number |
CAS 1025504-45-3
|
|||
| PubChem Compound ID | ||||
| ADReCS Drug ID | BADD_D02491 | |||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03698331) The Potential for Clinical Dependence and Withdrawal Symptoms Associated With Valbenazine. U.S. National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT03732534) Rollover Study for Continuing NBI-98854 Administration in Pediatric Subjects With Tourette Syndrome. U.S. National Institutes of Health. | |||
| REF 3 | VMAT2 Inhibitors and the Path to Ingrezza (Valbenazine). Prog Med Chem. 2018;57(1):87-111. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

