Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T48873
(Former ID: TTDS00118)
|
|||||
| Target Name |
Synaptic vesicle amine transporter (SLC18A2)
|
|||||
| Synonyms |
Vesicular amine transporter; Vesicular Monoamine Transporter; VMAT; VAT; Solute carrier family 18 member 2; SLC18A2; Monoamine transporter
Click to Show/Hide
|
|||||
| Gene Name |
SLC18A2
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 5 Target-related Diseases | + | ||||
| 1 | Choreiform disorder [ICD-11: 8A01] | |||||
| 2 | Dissociative neurological symptom disorder [ICD-11: 6B60] | |||||
| 3 | Essential hypertension [ICD-11: BA00] | |||||
| 4 | Hypertension [ICD-11: BA00-BA04] | |||||
| 5 | Dystonic disorder [ICD-11: 8A02] | |||||
| Function |
Involved in the ATP-dependent vesicular transport of biogenic amine neurotransmitters. Pumps cytosolic monoamines including dopamine, norepinephrine, serotonin, and histamine into synaptic vesicles. Requisite for vesicular amine storage prior to secretion via exocytosis.
Click to Show/Hide
|
|||||
| BioChemical Class |
Major facilitator superfamily
|
|||||
| UniProt ID | ||||||
| Sequence |
MALSELALVRWLQESRRSRKLILFIVFLALLLDNMLLTVVVPIIPSYLYSIKHEKNATEI
QTARPVHTASISDSFQSIFSYYDNSTMVTGNATRDLTLHQTATQHMVTNASAVPSDCPSE DKDLLNENVQVGLLFASKATVQLITNPFIGLLTNRIGYPIPIFAGFCIMFVSTIMFAFSS SYAFLLIARSLQGIGSSCSSVAGMGMLASVYTDDEERGNVMGIALGGLAMGVLVGPPFGS VLYEFVGKTAPFLVLAALVLLDGAIQLFVLQPSRVQPESQKGTPLTTLLKDPYILIAAGS ICFANMGIAMLEPALPIWMMETMCSRKWQLGVAFLPASISYLIGTNIFGILAHKMGRWLC ALLGMIIVGVSILCIPFAKNIYGLIAPNFGVGFAIGMVDSSMMPIMGYLVDLRHVSVYGS VYAIADVAFCMGYAIGPSAGGAIAKAIGFPWLMTIIGIIDILFAPLCFFLRSPPAKEEKM AILMDHNCPIKTKMYTQNNIQSYPIGEDEESESD Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T74J8E | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 7 Approved Drugs | + | ||||
| 1 | Alkavervir | Drug Info | Approved | High blood pressure | [2] | |
| 2 | Alseroxylon | Drug Info | Approved | Hypertension | [3] | |
| 3 | Deutetrabenazine | Drug Info | Approved | Tardive dyskinesia | [4] | |
| 4 | Reserpine | Drug Info | Approved | Hypertension | [5], [6] | |
| 5 | Tetrabenazine | Drug Info | Approved | Hyperkinetic movement disorder | [2], [7], [8], [9], [10], [11] | |
| 6 | Valbenazine Tosylate | Drug Info | Approved | Tardive dyskinesia | [12], [4] | |
| 7 | Ingrezza | Drug Info | Phase 4 | Tardive dyskinesia | [13] | |
| Clinical Trial Drug(s) | [+] 4 Clinical Trial Drugs | + | ||||
| 1 | AV 133 | Drug Info | Phase 3 | Alzheimer disease | [14] | |
| 2 | NBI-98854 | Drug Info | Phase 3 | Movement disorder | [15], [16] | |
| 3 | Lobeline | Drug Info | Phase 2 | Substance use disorder | [17] | |
| 4 | SOM3355 | Drug Info | Phase 2 | Huntington disease | [18] | |
| Mode of Action | [+] 5 Modes of Action | + | ||||
| Modulator | [+] 3 Modulator drugs | + | ||||
| 1 | Alkavervir | Drug Info | [19] | |||
| 2 | Deutetrabenazine | Drug Info | [19] | |||
| 3 | Valbenazine Tosylate | Drug Info | [19] | |||
| Blocker | [+] 3 Blocker drugs | + | ||||
| 1 | Alseroxylon | Drug Info | [1] | |||
| 2 | Reserpine | Drug Info | [20] | |||
| 3 | Tetrabenazine | Drug Info | [20], [21] | |||
| Antagonist | [+] 4 Antagonist drugs | + | ||||
| 1 | Ingrezza | Drug Info | [22] | |||
| 2 | Beta-methoxyamphetamine | Drug Info | [27] | |||
| 3 | METHYLENEDIOXYMETHAMPHETAMINE | Drug Info | [28] | |||
| 4 | MMDA | Drug Info | [29] | |||
| Enhancer | [+] 1 Enhancer drugs | + | ||||
| 1 | AV 133 | Drug Info | [23] | |||
| Inhibitor | [+] 5 Inhibitor drugs | + | ||||
| 1 | NBI-98854 | Drug Info | [24] | |||
| 2 | Lobeline | Drug Info | [25] | |||
| 3 | SOM3355 | Drug Info | [26] | |||
| 4 | [11C]DTBZ | Drug Info | [30] | |||
| 5 | [125I]7-azido-8-iodoketanserine | Drug Info | [31] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Solute carrier family 22 member 1 (SLC22A1) | 23.881 (32/134) | 5.00E-03 |
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
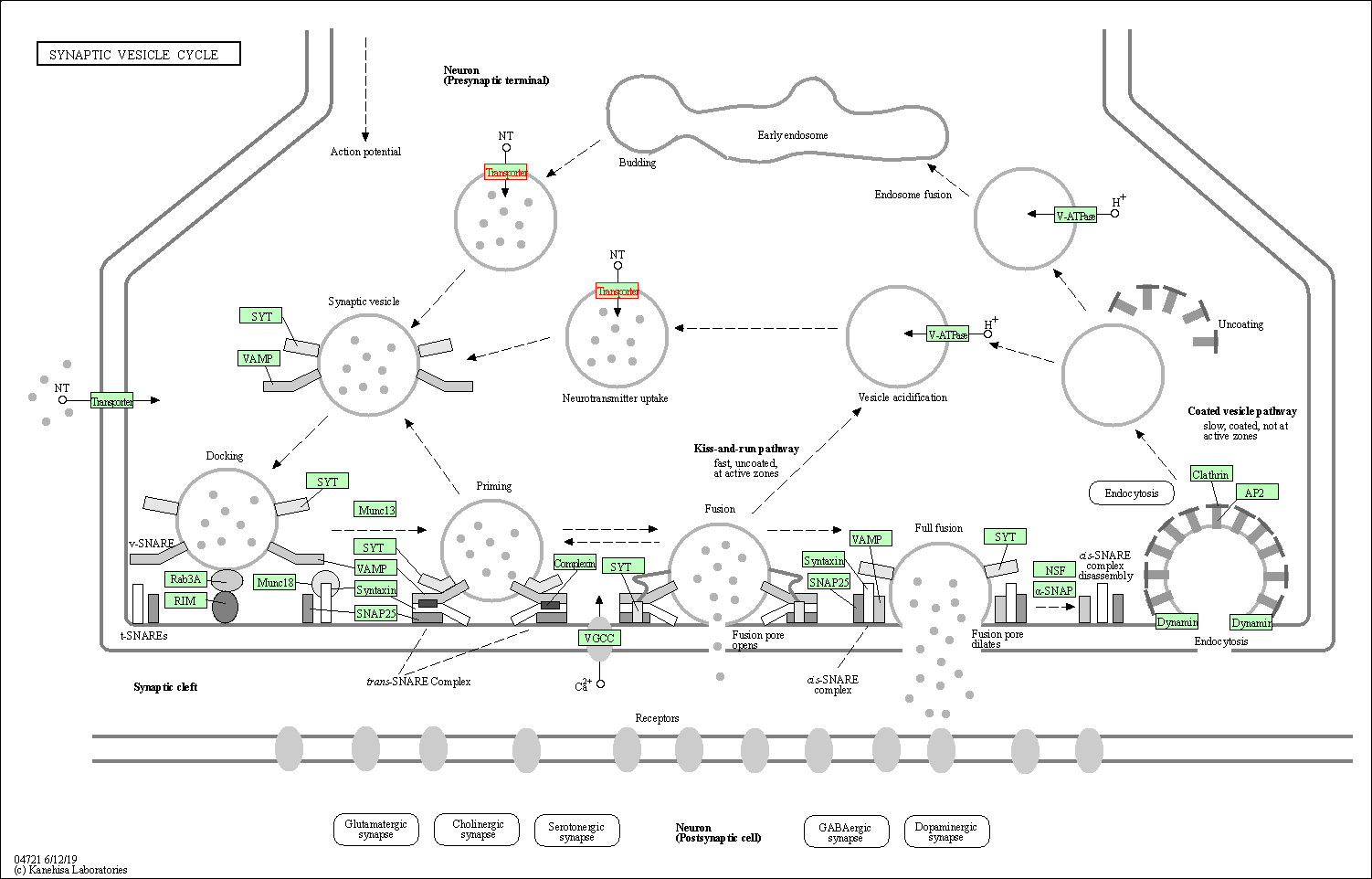
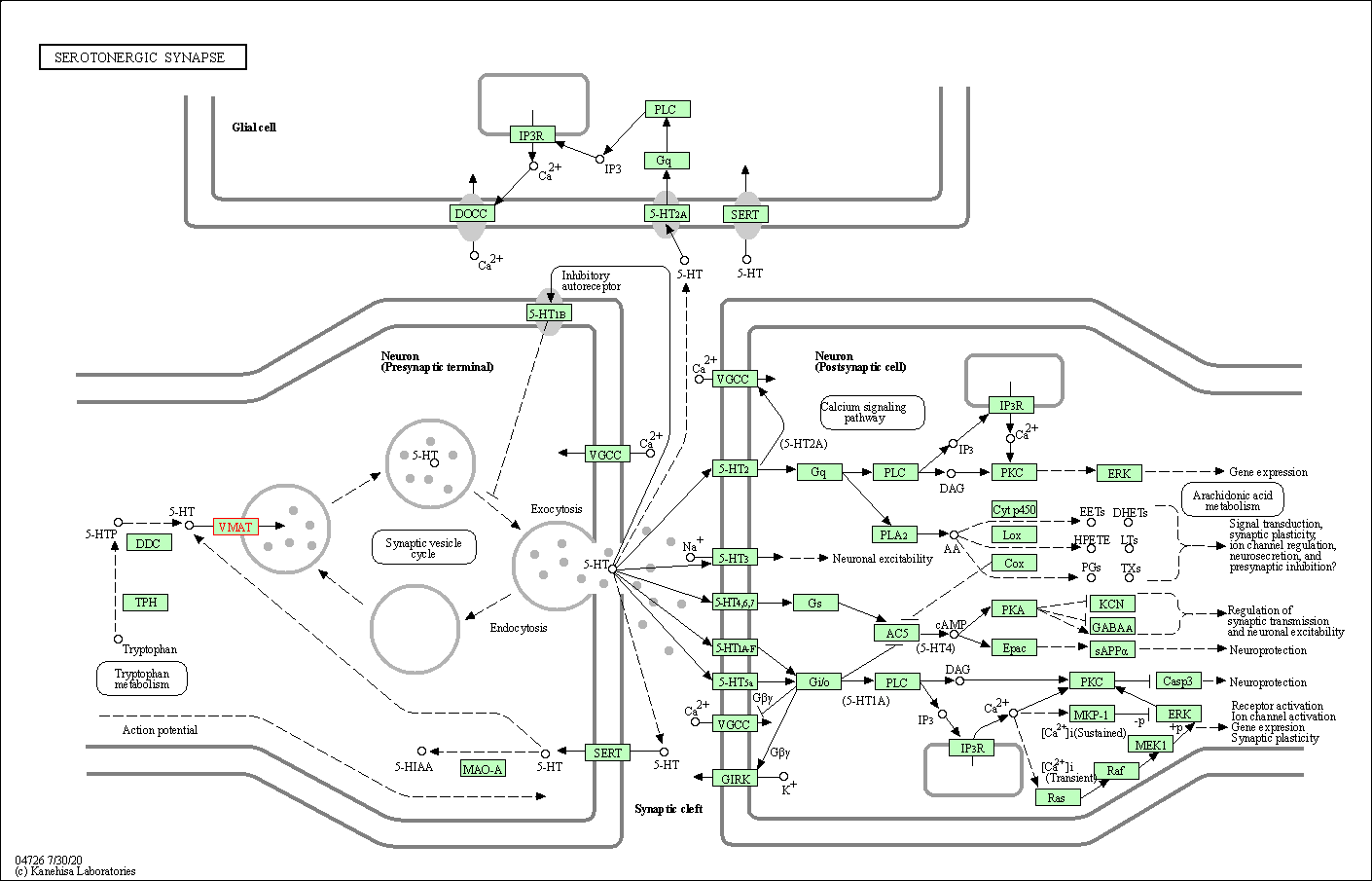
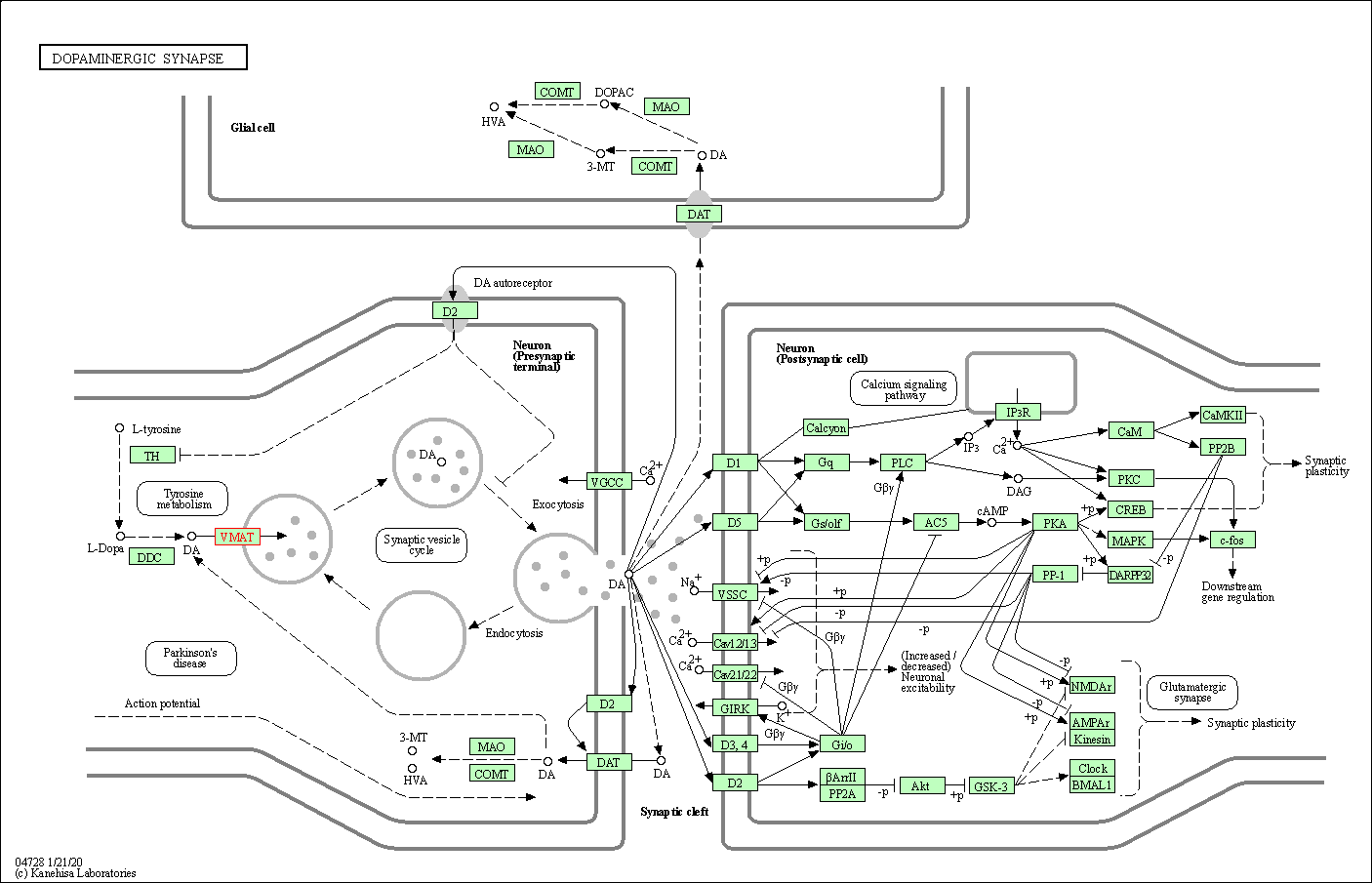
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Synaptic vesicle cycle | hsa04721 | Affiliated Target |

|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Serotonergic synapse | hsa04726 | Affiliated Target |

|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Dopaminergic synapse | hsa04728 | Affiliated Target |

|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Degree | 3 | Degree centrality | 3.22E-04 | Betweenness centrality | 1.99E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.99E-01 | Radiality | 1.34E+01 | Clustering coefficient | 3.33E-01 |
| Neighborhood connectivity | 1.43E+01 | Topological coefficient | 3.50E-01 | Eccentricity | 13 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 7 KEGG Pathways | + | ||||
| 1 | Synaptic vesicle cycle | |||||
| 2 | Serotonergic synapse | |||||
| 3 | Dopaminergic synapse | |||||
| 4 | Parkinson's disease | |||||
| 5 | Cocaine addiction | |||||
| 6 | Amphetamine addiction | |||||
| 7 | Alcoholism | |||||
| Panther Pathway | [+] 8 Panther Pathways | + | ||||
| 1 | Adrenaline and noradrenaline biosynthesis | |||||
| 2 | 5HT1 type receptor mediated signaling pathway | |||||
| 3 | 5HT2 type receptor mediated signaling pathway | |||||
| 4 | 5HT3 type receptor mediated signaling pathway | |||||
| 5 | 5HT4 type receptor mediated signaling pathway | |||||
| 6 | Dopamine receptor mediated signaling pathway | |||||
| 7 | Nicotine pharmacodynamics pathway | |||||
| 8 | CCKR signaling map ST | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | Norepinephrine Neurotransmitter Release Cycle | |||||
| 2 | Na+/Cl- dependent neurotransmitter transporters | |||||
| WikiPathways | [+] 4 WikiPathways | + | ||||
| 1 | Dopaminergic Neurogenesis | |||||
| 2 | Synaptic Vesicle Pathway | |||||
| 3 | Neurotransmitter Release Cycle | |||||
| 4 | Nicotine Activity on Dopaminergic Neurons | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34. | |||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 3 | Drug information of Alseroxylon, 2008. eduDrugs. | |||||
| REF 4 | 2017 FDA drug approvals.Nat Rev Drug Discov. 2018 Feb;17(2):81-85. | |||||
| REF 5 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4823). | |||||
| REF 6 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 009838. | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4834). | |||||
| REF 8 | 2008 FDA drug approvals. Nat Rev Drug Discov. 2009 Feb;8(2):93-6. | |||||
| REF 9 | The druggable genome: Evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu Rev Pharmacol Toxicol. 2014;54:9-26. | |||||
| REF 10 | Role of tetrabenazine for Huntington's disease-associated chorea. Ann Pharmacother. 2010 Jun;44(6):1080-9. | |||||
| REF 11 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 021894. | |||||
| REF 12 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2018 | |||||
| REF 13 | ClinicalTrials.gov (NCT03698331) The Potential for Clinical Dependence and Withdrawal Symptoms Associated With Valbenazine. U.S. National Institutes of Health. | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800027191) | |||||
| REF 15 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8694). | |||||
| REF 16 | ClinicalTrials.gov (NCT02274558) A Phase 3 Study of NBI-98854 for the Treatment of Tardive Dyskinesia. U.S. National Institutes of Health. | |||||
| REF 17 | Effects of VMAT2 inhibitors lobeline and GZ-793A on methamphetamine-induced changes in dopamine release, metabolism and synthesis in vivo. J Neurochem. 2013 Oct;127(2):187-98. | |||||
| REF 18 | ClinicalTrials.gov (NCT03575676) Efficacy and Safety of SOM3355 in Huntington's Disease Chorea. U.S. National Institutes of Health. | |||||
| REF 19 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 20 | Dopamine signaling is required for depolarization-induced slow current in cerebellar Purkinje cells. J Neurosci. 2009 Jul 1;29(26):8530-8. | |||||
| REF 21 | 11C-dihydrotetrabenazine PET of the pancreas in subjects with long-standing type 1 diabetes and in healthy controls. J Nucl Med. 2009 Mar;50(3):382-9. | |||||
| REF 22 | VMAT2 Inhibitors and the Path to Ingrezza (Valbenazine). Prog Med Chem. 2018;57(1):87-111. | |||||
| REF 23 | Brain imaging of vesicular monoamine transporter type 2 in healthy aging subjects by 18F-FP-(+)-DTBZ PET. PLoS One. 2013 Sep 30;8(9):e75952. | |||||
| REF 24 | NBI-98854, a selective monoamine transport inhibitor for the treatment of tardive dyskinesia: A randomized, double-blind, placebo-controlled study. Mov Disord. 2015 Oct;30(12):1681-7. | |||||
| REF 25 | Design, synthesis and interaction at the vesicular monoamine transporter-2 of lobeline analogs: potential pharmacotherapies for the treatment of psychostimulant abuse. Curr Top Med Chem. 2011;11(9):1103-27. | |||||
| REF 26 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 27 | Differential behavioural and neurochemical effects of para-methoxyamphetamine and 3,4-methylenedioxymethamphetamine in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2000 Aug;24(6):955-77. | |||||
| REF 28 | The origin of MDMA (ecstasy) revisited: the true story reconstructed from the original documents. Addiction. 2006 Sep;101(9):1241-5. | |||||
| REF 29 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 30 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1012). | |||||
| REF 31 | Peptide mapping of the [125I]Iodoazidoketanserin and [125I]2-N-[(3'-iodo-4'-azidophenyl)propionyl]tetrabenazine binding sites for the synaptic vesicle monoamine transporter. J Biol Chem. 1997 Oct 10;272(41):26049-55. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

