Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DES36M
|
|||
| Drug Name |
Q301
|
|||
| Synonyms |
Boc-N-Me-Val-OH; 45170-31-8; Boc-N-methyl-L-valine; N-Boc-N-methyl-L-valine; Boc-MeVal-OH; (S)-2-((tert-Butoxycarbonyl)(methyl)amino)-3-methylbutanoic acid; Boc-N-a-methyl-L-valine; L-Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-; MFCD00038760; n-(tert-butoxycarbonyl)-n-methyl-l-valine; N-Boc-N-methylvaline; (2S)-3-methyl-2-[methyl-[(2-methylpropan-2-yl)oxycarbonyl]amino]butanoic acid; PubChem12254; Boc-N-Me-L-Val-OH; Boc-Nalpha-methyl-L-valine; Boc-N-; A-Methyl-L-valine; N-Boc-N-Methyl-L-Val-OH; SCHEMBL59435; DTXSID10426676; ZINC2391126; ANW-41482; AKOS015836686; AKOS015905243; AC-8571; AM82390; AT-5684; CS-W008976; AS-15695; BP-21374; AB0017502; Boc-N-Me-Val-OH, >=99.0% (TLC); DB-038153; 170B318; J-300310; N-alpha-t-Butyloxycarbonyl-N-alpha-methyl-L-valine; Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-; (2S)-(tert-Butoxycarbonyl-methyl-amino)-3-methyl-butyric acid; (s)-2-(t-butoxycarbonyl(methyl)amino)-3-methylbutanoic acid; (s)-2-(tert-butoxycarbonyl(methyl)amino)-3-methylbutanoic acid; (S)-2-(tert-butoxycarbonyl-methyl-amino)-3-methyl-butyric acid; (2S)-2-[[(tert-butoxy)carbonyl](methyl)amino]-3-methyl butanoic acid; (2S)-2-[[(tert-Butoxy)carbonyl](methyl)amino]-3-methylbutanoic acid; (2S)-3-methyl-2-[methyl-[(2-methylpropan-2-yl)oxycarbonyl]amino]butanoicacid
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Atopic dermatitis [ICD-11: EA80; ICD-10: L20] | Phase 2 | [1] | |
| Company |
Qurient
|
|||
| Structure |
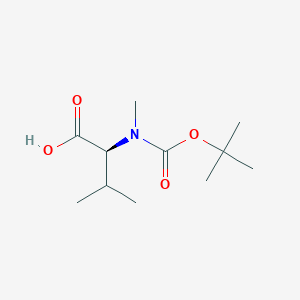 |
Download2D MOL |
||
| Formula |
C11H21NO4
|
|||
| Canonical SMILES |
CC(C)C(C(=O)O)N(C)C(=O)OC(C)(C)C
|
|||
| InChI |
1S/C11H21NO4/c1-7(2)8(9(13)14)12(6)10(15)16-11(3,4)5/h7-8H,1-6H3,(H,13,14)/t8-/m0/s1
|
|||
| InChIKey |
XPUAXAVJMJDPDH-QMMMGPOBSA-N
|
|||
| CAS Number |
CAS 45170-31-8
|
|||
| PubChem Compound ID | ||||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | Arachidonate 5-lipoxygenase (5-LOX) | Target Info | Inhibitor | [2] |
| BioCyc | Aspirin-triggered lipoxin biosynthesis | |||
| Resolvin D biosynthesis | ||||
| Leukotriene biosynthesis | ||||
| Lipoxin biosynthesis | ||||
| Aspirin triggered resolvin D biosynthesis | ||||
| Aspirin triggered resolvin E biosynthesis | ||||
| KEGG Pathway | Arachidonic acid metabolism | |||
| Metabolic pathways | ||||
| Serotonergic synapse | ||||
| Ovarian steroidogenesis | ||||
| Toxoplasmosis | ||||
| NetPath Pathway | IL4 Signaling Pathway | |||
| Pathwhiz Pathway | Arachidonic Acid Metabolism | |||
| WikiPathways | Vitamin D Receptor Pathway | |||
| Arachidonic acid metabolism | ||||
| Eicosanoid Synthesis | ||||
| Selenium Micronutrient Network | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT03571620) Safety and Efficacy Study of Q301 in Mild to Moderate Adolescents and Adults Atopic Dermatitis Patients. U.S. National Institutes of Health. | |||
| REF 2 | Clinical pipeline report, company report or official report of Qurient. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

