Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
DF1T2W
|
|||
| Drug Name |
GS-5718
|
|||
| Synonyms |
920036-34-6; tert-Butyl 3-iodo-1H-pyrazolo[3,4-b]pyridine-1-carboxylate; 1-BOC-3-IODO-1H-PYRAZOLO[3,4-B]PYRIDINE; tert-butyl 3-iodopyrazolo[3,4-b]pyridine-1-carboxylate; SCHEMBL3606964; OKKLWSNHFSFXEM-UHFFFAOYSA-N; AMY21637; MFCD13183727; AKOS022178093; GS-5718; PB26872; CS-0048281; FT-0702852; A860172; tert-Butyl3-iodo-1H-pyrazolo[3,4-b]pyridine-1-carboxylate; 1H-Pyrazolo[3,4-b]pyridine-1-carboxylicacid,3-iodo-,1,1-diMethylethylester; 1H-PYRAZOLO[3,4-B]PYRIDINE-1-CARBOXYLIC ACID, 3-IODO-, 1,1-DIMETHYLETHYLESTER
Click to Show/Hide
|
|||
| Drug Type |
Small molecule
|
|||
| Indication | Rheumatoid arthritis [ICD-11: FA20; ICD-9: 729] | Phase 2 | [1] | |
| Cutaneous lupus erythematosus [ICD-11: EB5Z; ICD-10: L80-L99, L93; ICD-9: 710] | Phase 1 | [2] | ||
| Company |
Gilead
|
|||
| Structure |
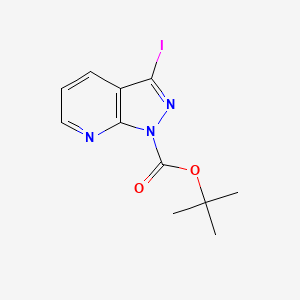 |
Download2D MOL |
||
| Formula |
C11H12IN3O2
|
|||
| Canonical SMILES |
CC(C)(C)OC(=O)N1C2=C(C=CC=N2)C(=N1)I
|
|||
| InChI |
InChI=1S/C11H12IN3O2/c1-11(2,3)17-10(16)15-9-7(8(12)14-15)5-4-6-13-9/h4-6H,1-3H3
|
|||
| InChIKey |
OKKLWSNHFSFXEM-UHFFFAOYSA-N
|
|||
| PubChem Compound ID | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | ClinicalTrials.gov (NCT05165771) A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase 2 Proof-of-Concept Study to Evaluate Safety, Tolerability, and Efficacy of GS-5718 on Background Therapy With Conventional Synthetic Disease-modifying Antirheumatic Drug(s) (csDMARDs) in Participants With Active Rheumatoid Arthritis Who Have an Inadequate Response to Biologic DMARD(s) Treatment. U.S.National Institutes of Health. | |||
| REF 2 | ClinicalTrials.gov (NCT04809623) A Randomized, Blinded, Placebo-Controlled, Phase 1b Study of GS-5718 in Subjects With Cutaneous Lupus Erythematosus (CLE). U.S.National Institutes of Health. | |||
| REF 3 | IRAK4 degrader to take on innate immunity. Nat Biotechnol. 2020 Nov;38(11):1221-1223. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

