Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T61698
(Former ID: TTDR00141)
|
|||||
| Target Name |
Interleukin-2 (IL2)
|
|||||
| Synonyms |
TCGF; T-cell growth factor; IL-2; Aldesleukin
Click to Show/Hide
|
|||||
| Gene Name |
IL2
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Multiple sclerosis [ICD-11: 8A40] | |||||
| 2 | Renal cell carcinoma [ICD-11: 2C90] | |||||
| Function |
Produced by T-cells in response to antigenic or mitogenic stimulation, this protein is required for T-cell proliferation and other activities crucial to regulation of the immune response. Can stimulate B-cells, monocytes, lymphokine-activated killer cells, natural killer cells, and glioma cells.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine: interleukin
|
|||||
| UniProt ID | ||||||
| Sequence |
MYRMQLLSCIALSLALVTNSAPTSSSTKKTQLQLEHLLLDLQMILNGINNYKNPKLTRML
TFKFYMPKKATELKHLQCLEEELKPLEEVLNLAQSKNFHLRPRDLISNINVIVLELKGSE TTFMCEYADETATIVEFLNRWITFCQSIISTLT Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A03025 | |||||
| HIT2.0 ID | T34DZ7 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 2 Approved Drugs | + | ||||
| 1 | Aldesleukin | Drug Info | Approved | Renal cell carcinoma | [2] | |
| 2 | Daclizumab | Drug Info | Approved | Multiple sclerosis | [3] | |
| Clinical Trial Drug(s) | [+] 16 Clinical Trial Drugs | + | ||||
| 1 | AIR-insulin | Drug Info | Phase 3 | Diabetic complication | [4] | |
| 2 | TG-4010 | Drug Info | Phase 2/3 | Bipolar disorder | [5] | |
| 3 | APN-301 | Drug Info | Phase 2 | Melanoma | [6] | |
| 4 | BNZ-1 | Drug Info | Phase 2 | Cutaneous T-cell lymphoma | [7] | |
| 5 | Carboxyamidotriazole orotate | Drug Info | Phase 2 | Glioblastoma multiforme | [8] | |
| 6 | Human interleukin-2 | Drug Info | Phase 2 | Renal cell carcinoma | [9] | |
| 7 | IL-2/CD40L-expressing leukemia vaccine | Drug Info | Phase 2 | Chronic lymphocytic leukaemia | [10] | |
| 8 | L19-IL-2 fusion protein | Drug Info | Phase 2 | Lymphoma | [11] | |
| 9 | Leuvectin | Drug Info | Phase 2 | Melanoma | [12] | |
| 10 | Thymoctonan | Drug Info | Phase 2 | Immune System disease | [13] | |
| 11 | IL-2 XL | Drug Info | Phase 1/2 | Renal cell carcinoma | [14] | |
| 12 | IL-2/gene-modified lymphocytes | Drug Info | Phase 1/2 | Ovarian cancer | [15] | |
| 13 | Medusa IL-2 | Drug Info | Phase 1/2 | Solid tumour/cancer | [16] | |
| 14 | ASP9801 | Drug Info | Phase 1 | Solid tumour/cancer | [17] | |
| 15 | SAR444245 | Drug Info | Phase 1 | Solid tumour/cancer | [18] | |
| 16 | VCL-1M01 | Drug Info | Phase 1 | Melanoma | [19] | |
| Discontinued Drug(s) | [+] 6 Discontinued Drugs | + | ||||
| 1 | Nuleusin | Drug Info | Discontinued in Phase 3 | Melanoma | [20] | |
| 2 | PMI-001 | Drug Info | Discontinued in Phase 3 | Lupus | [21] | |
| 3 | BIWB-1 | Drug Info | Discontinued in Phase 2 | Melanoma | [22] | |
| 4 | VLTS-587 | Drug Info | Discontinued in Phase 2 | Lung cancer | [23] | |
| 5 | Roquinimex | Drug Info | Discontinued in Phase 1 | Rheumatoid arthritis | [24] | |
| 6 | TG-1024 | Drug Info | Discontinued in Phase 1 | Solid tumour/cancer | [25] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | VPM-4-001 | Drug Info | Preclinical | Prostate cancer | [26] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Modulator | [+] 19 Modulator drugs | + | ||||
| 1 | Aldesleukin | Drug Info | [1] | |||
| 2 | Daclizumab | Drug Info | [3] | |||
| 3 | AIR-insulin | Drug Info | [27] | |||
| 4 | TG-4010 | Drug Info | [5] | |||
| 5 | APN-301 | Drug Info | [28] | |||
| 6 | Human interleukin-2 | Drug Info | [31] | |||
| 7 | Leuvectin | Drug Info | [34] | |||
| 8 | Thymoctonan | Drug Info | [35] | |||
| 9 | IL-2 XL | Drug Info | [16] | |||
| 10 | IL-2/gene-modified lymphocytes | Drug Info | [36] | |||
| 11 | Medusa IL-2 | Drug Info | [37] | |||
| 12 | VCL-1M01 | Drug Info | [40] | |||
| 13 | VLTS-587 | Drug Info | [44] | |||
| 14 | Roquinimex | Drug Info | [45] | |||
| 15 | TG-1024 | Drug Info | [46] | |||
| 16 | IL-2 antibody (anti-tumor) | Drug Info | [48] | |||
| 17 | interleukin-2, Roussel Uclaf | Drug Info | [49] | |||
| 18 | TG-1031 | Drug Info | [51] | |||
| 19 | TG-2001 | Drug Info | [52] | |||
| Inhibitor | [+] 5 Inhibitor drugs | + | ||||
| 1 | BNZ-1 | Drug Info | [29] | |||
| 2 | Carboxyamidotriazole orotate | Drug Info | [30] | |||
| 3 | PMI-001 | Drug Info | [42] | |||
| 4 | SP2456 | Drug Info | [50] | |||
| 5 | SP4160 | Drug Info | [50] | |||
| Agonist | [+] 1 Agonist drugs | + | ||||
| 1 | Nuleusin | Drug Info | [41] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: SP2456 | Ligand Info | |||||
| Structure Description | Low Micromolar Small Molecule Inhibitor of IL-2 | PDB:1PW6 | ||||
| Method | X-ray diffraction | Resolution | 2.60 Å | Mutation | No | [53] |
| PDB Sequence |
STKKTQLQLE
15 HLLLDLQMIL25 NGINNYKNPK35 LTRMLTFKFY45 MPKKATELKH55 LQCLEEELKP 65 LEEVLNLAQR81 PRDLISNINV91 IVLELKGSET101 TFMCEYADET111 ATIVEFLNRW 121 ITFCQSIIST131 L
|
|||||
|
|
||||||
| Ligand Name: SP4160 | Ligand Info | |||||
| Structure Description | Structure of SP4160 Bound to IL-2 V69A | PDB:1QVN | ||||
| Method | X-ray diffraction | Resolution | 2.70 Å | Mutation | Yes | [54] |
| PDB Sequence |
SSSTKKTQLQ
13 LEHLLLDLQM23 ILNGINNYKN33 PKLTRMLTFK43 FYMPKKATEL53 KHLQCLEEEL 63 KPLEEALNLA73 QRPRDLISNI89 NVIVLELKGS99 ETTFMCEYAD109 ETATIVEFLN 119 RWITFCQSII129 STL
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
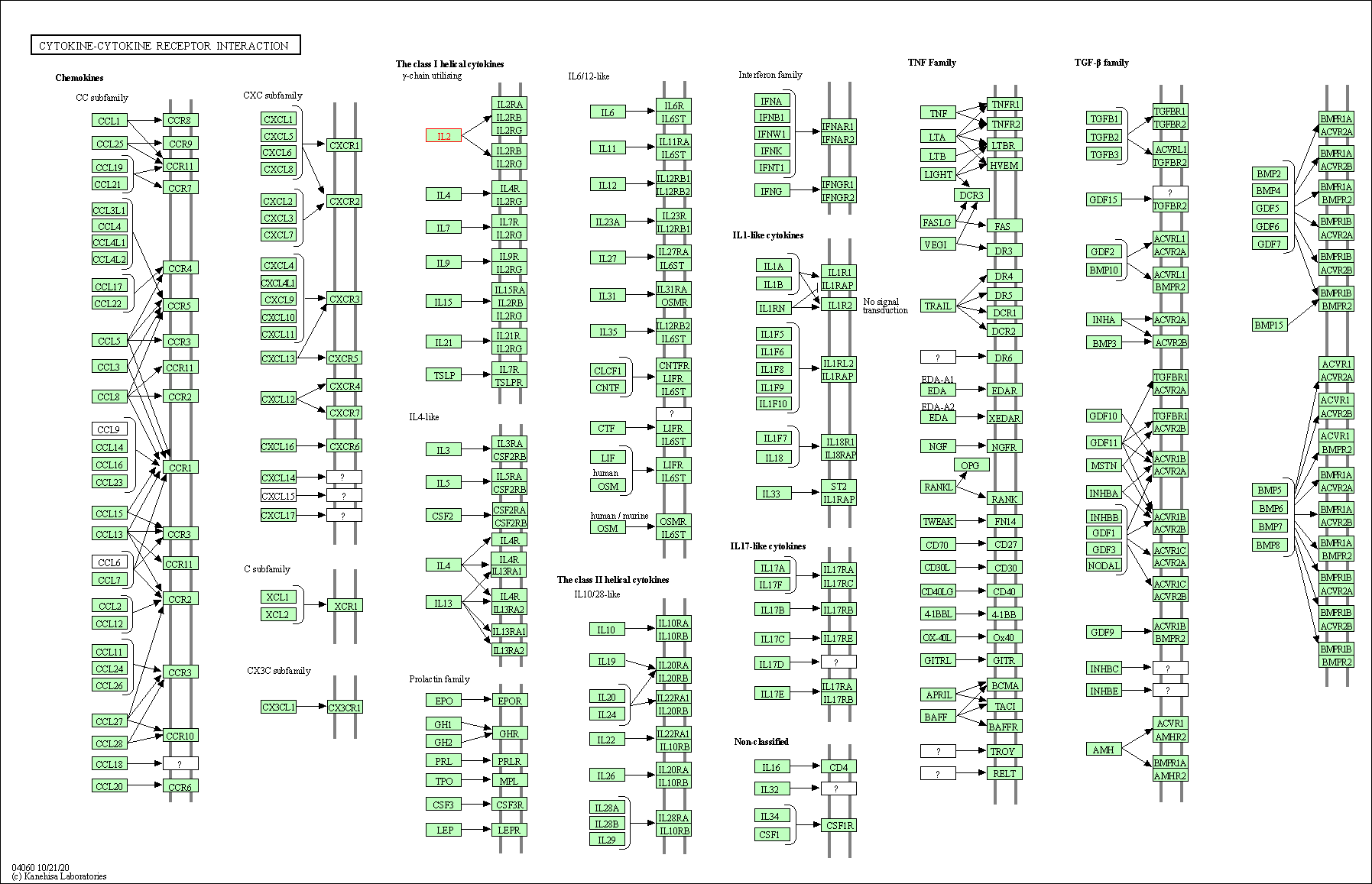
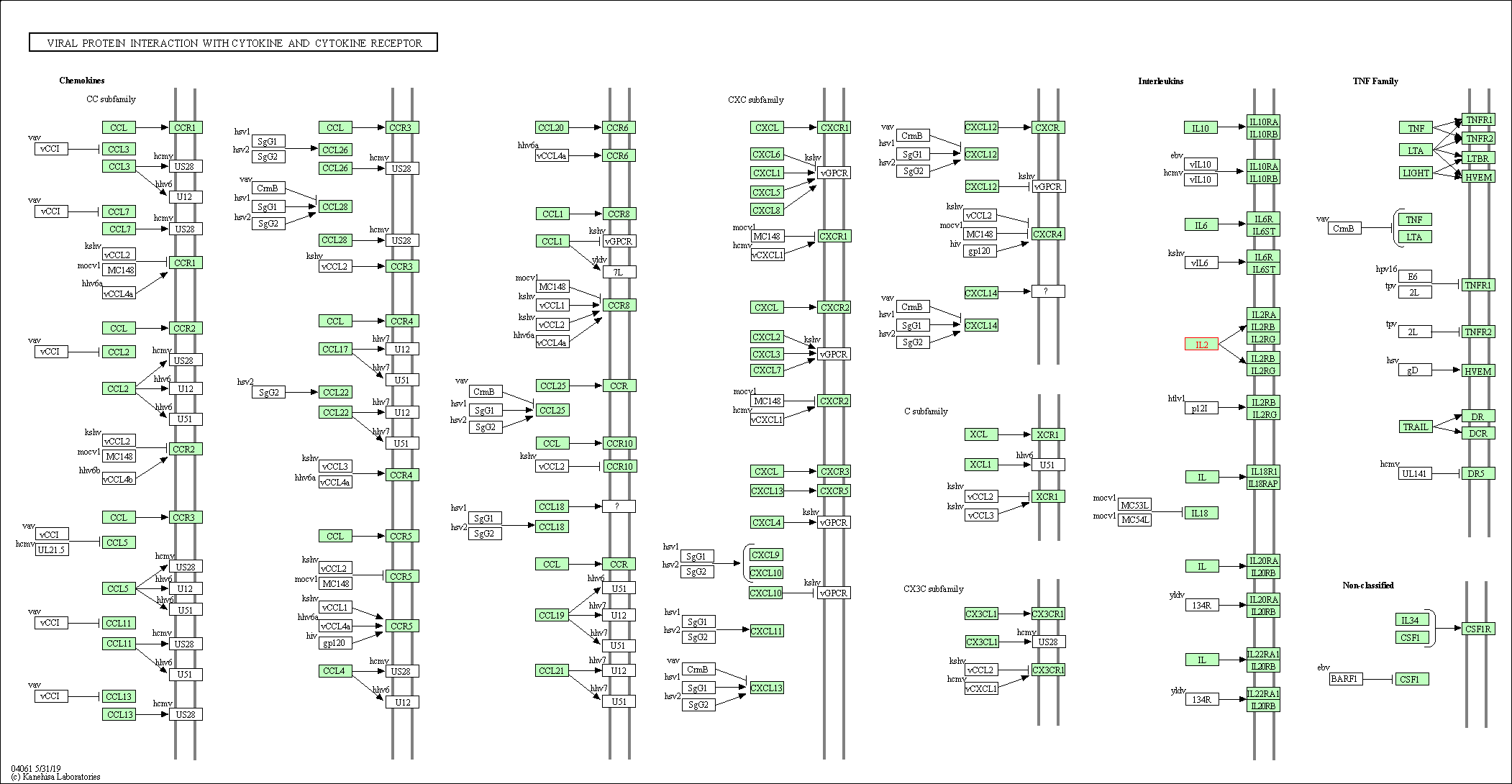
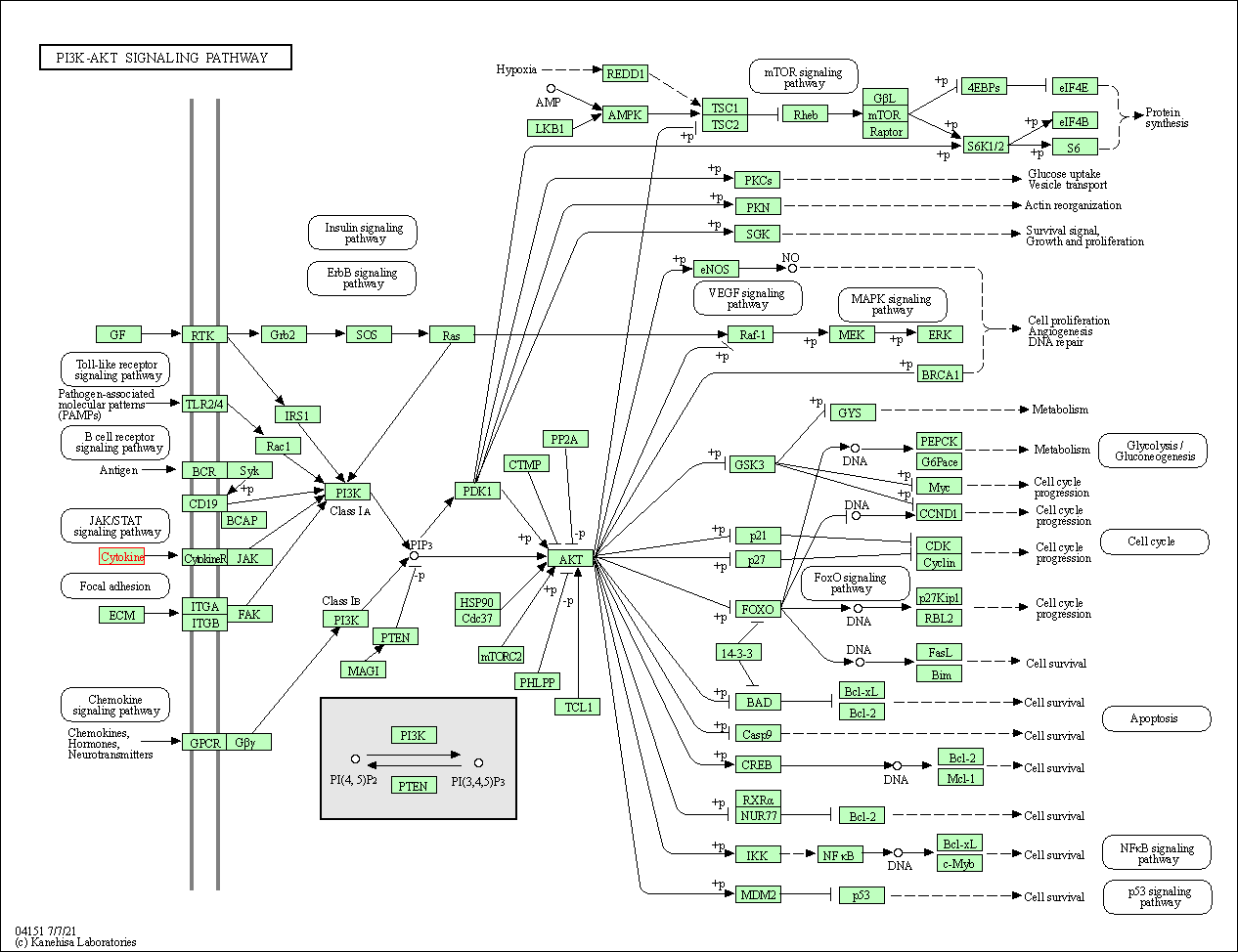
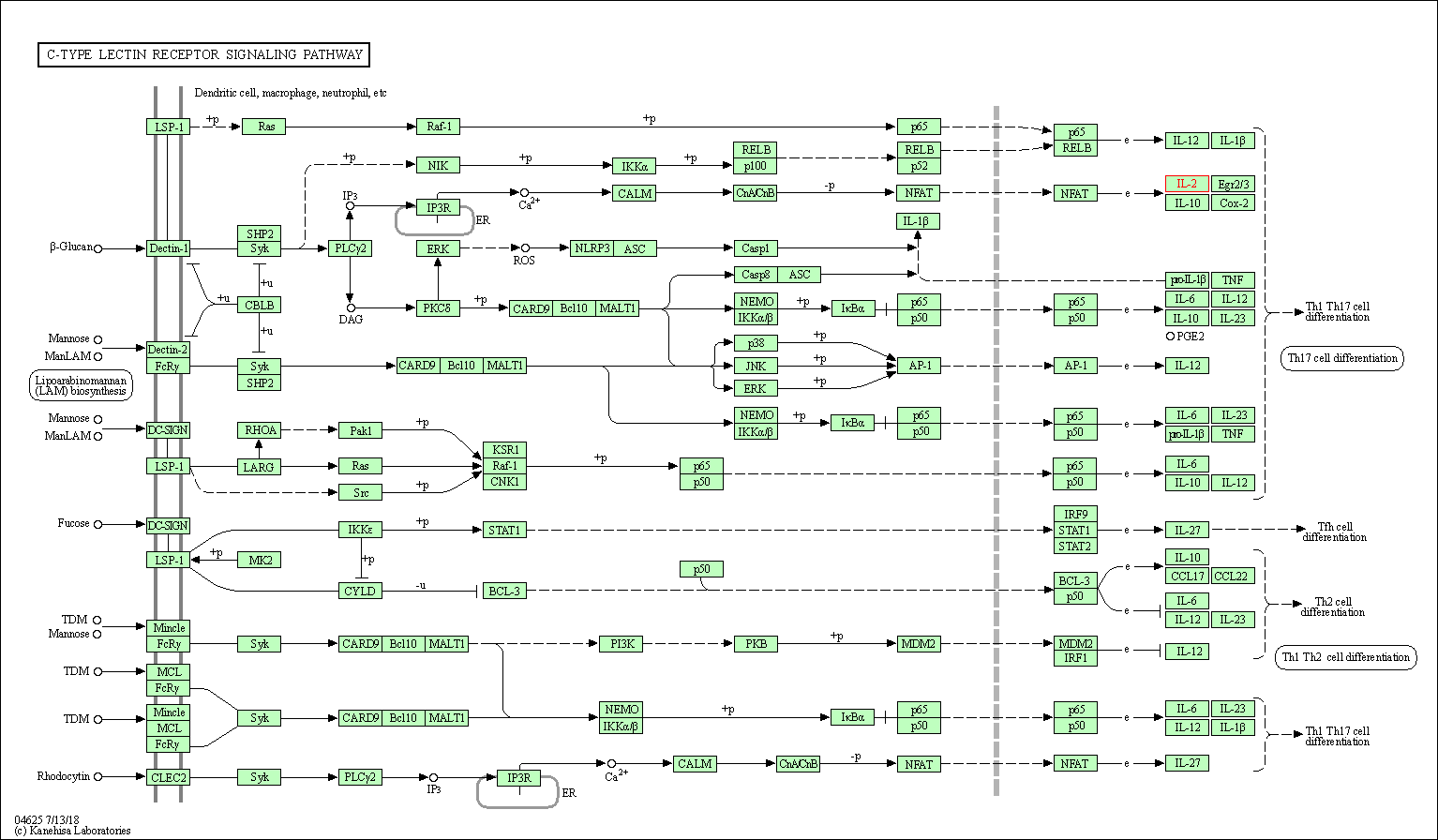
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Viral protein interaction with cytokine and cytokine receptor | hsa04061 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| C-type lectin receptor signaling pathway | hsa04625 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
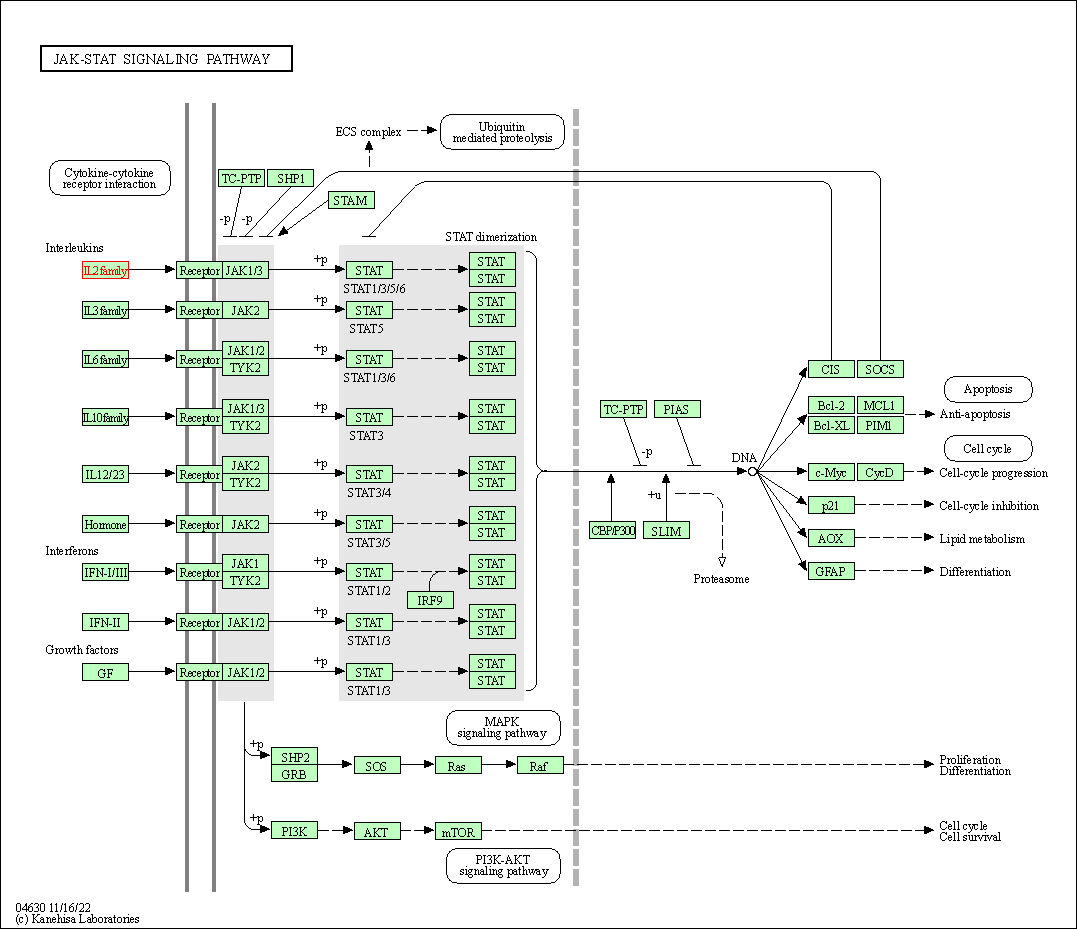
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
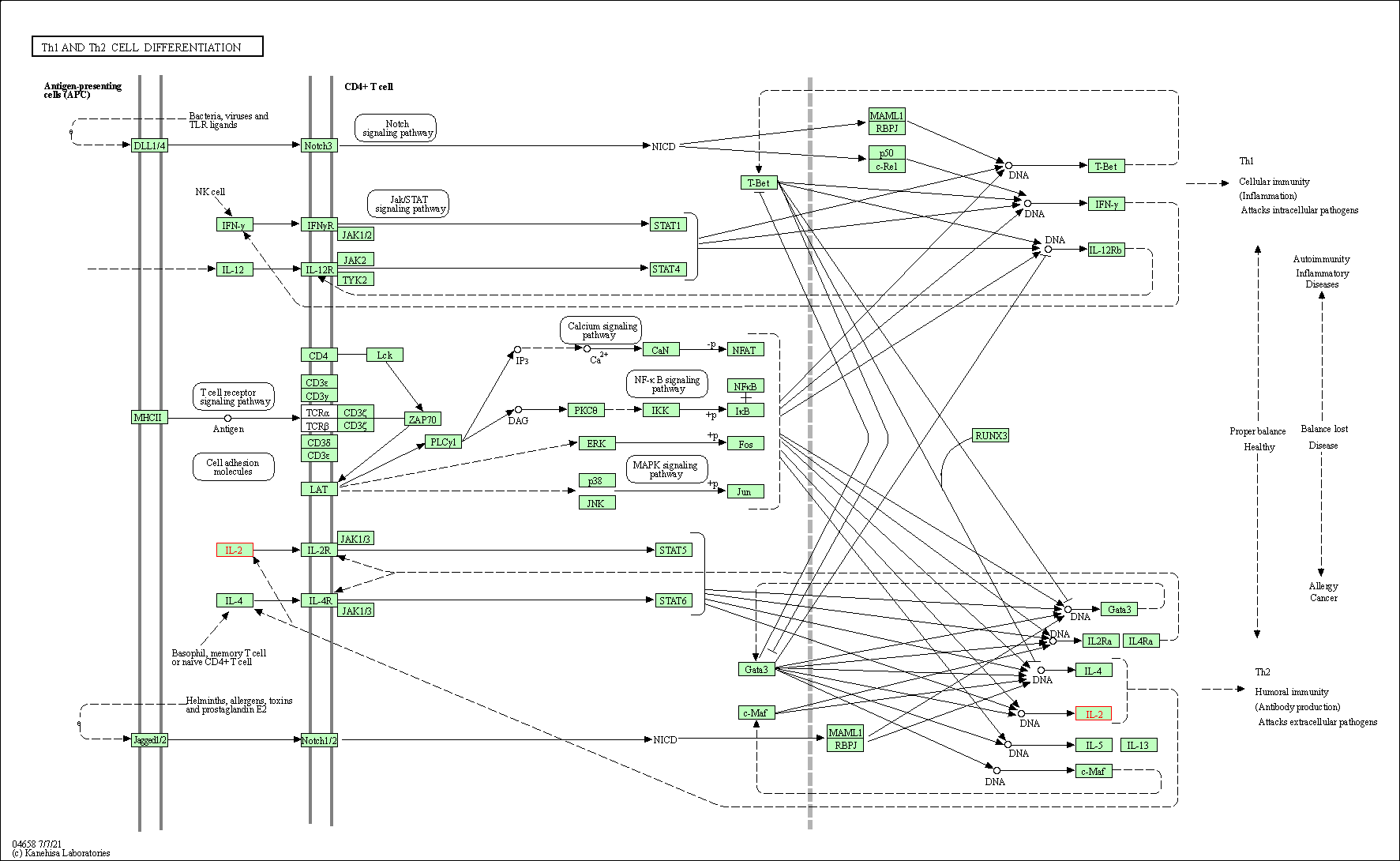
| Th1 and Th2 cell differentiation | hsa04658 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
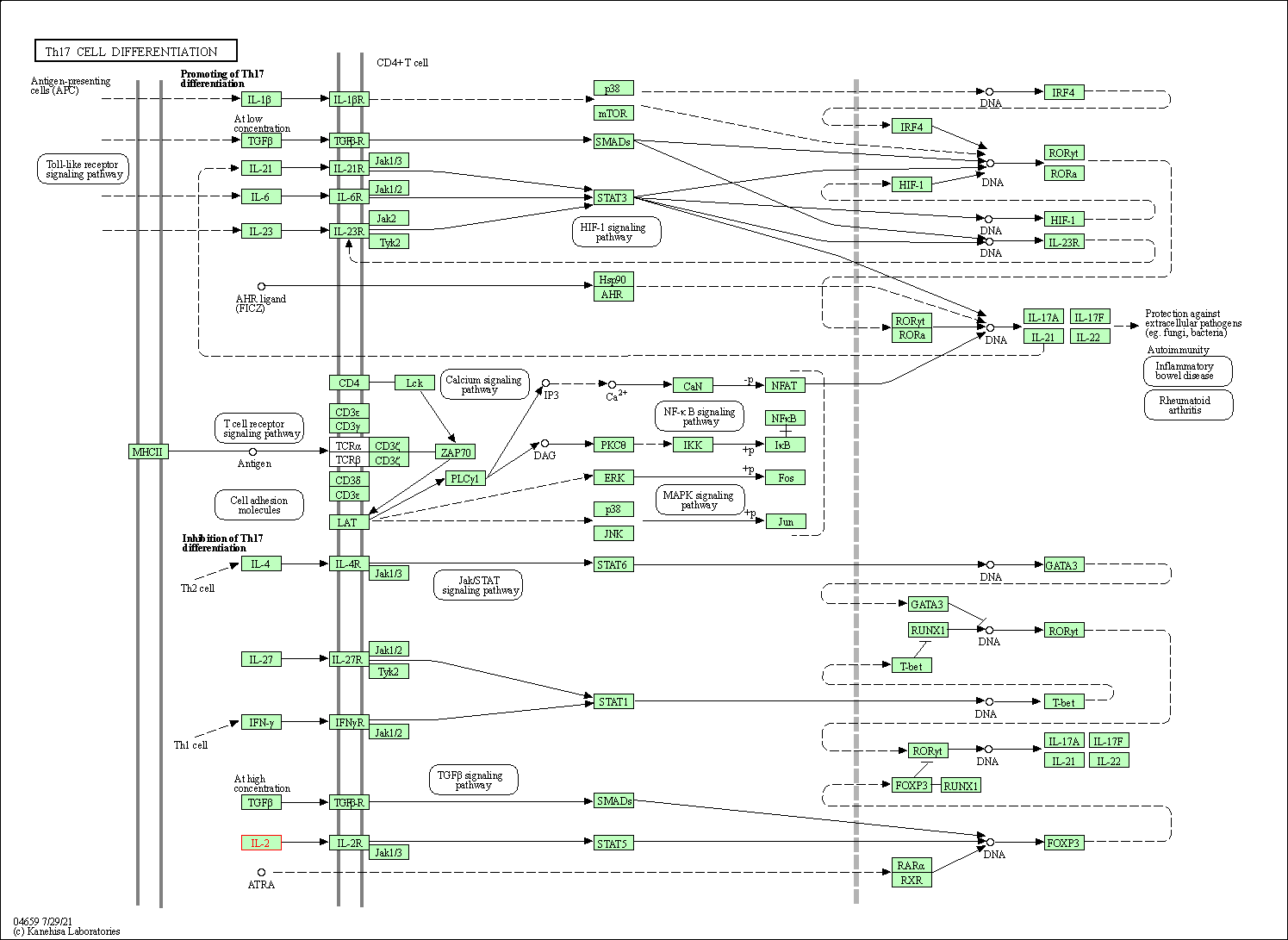
| Th17 cell differentiation | hsa04659 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
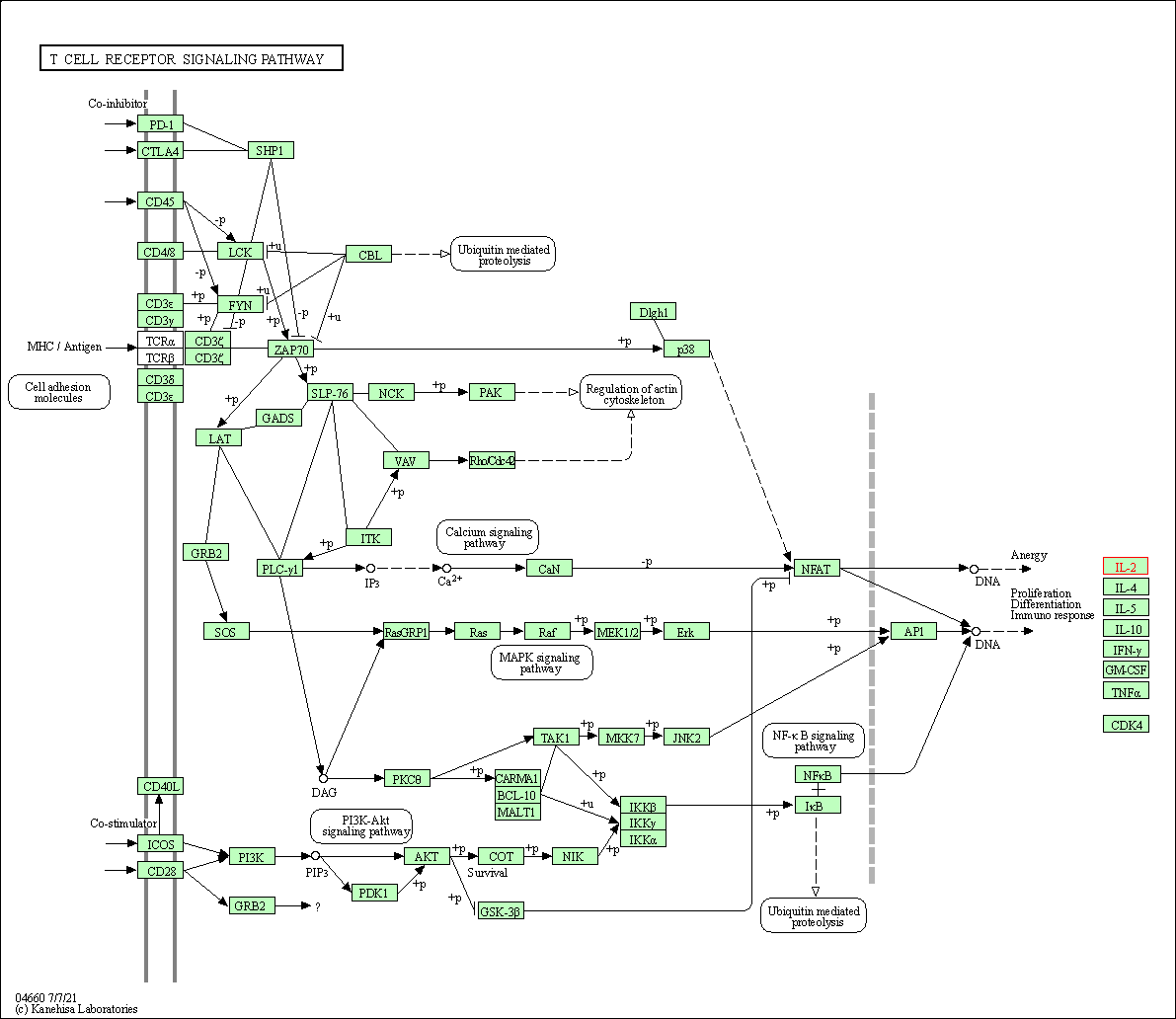
| T cell receptor signaling pathway | hsa04660 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Intestinal immune network for IgA production | hsa04672 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 27 | Degree centrality | 2.90E-03 | Betweenness centrality | 1.89E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.47E-01 | Radiality | 1.43E+01 | Clustering coefficient | 2.48E-01 |
| Neighborhood connectivity | 4.74E+01 | Topological coefficient | 7.47E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 3 | 2016 FDA drug approvals. Nat Rev Drug Discov. 2017 Feb 2;16(2):73-76. | |||||
| REF 4 | Clinical pipeline report, company report or official report of Eli Lilly. | |||||
| REF 5 | A phase II study of Tg4010 (Mva-Muc1-Il2) in association with chemotherapy in patients with stage III/IV Non-small cell lung cancer. J Thorac Oncol. 2008 Jul;3(7):735-44. | |||||
| REF 6 | ClinicalTrials.gov (NCT00590824) Pilot hu14.18-IL2 in Resectable Recurrent Stage III or Stage IV Melanoma. U.S. National Institutes of Health. | |||||
| REF 7 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 8 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800032169) | |||||
| REF 9 | Clinical pipeline report, company report or official report of Immunservice GmbH. | |||||
| REF 10 | ClinicalTrials.gov (NCT01604031) Treatment of B-CLL With Autologous IL2 and CD40 Ligand-Expressing Tumor Cells + Lenalidomide. U.S. National Institutes of Health. | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026492) | |||||
| REF 12 | ClinicalTrials.gov (NCT00004050) Leuvectin Followed By Surgery in Treating Patients With Stage II or Stage III Prostate Cancer. U.S. National Institutes of Health. | |||||
| REF 13 | ClinicalTrials.gov (NCT00002435) A Study of Thymic Humoral Factor (THF Gamma 2) in HIV-Infected Patients. U.S. National Institutes of Health. | |||||
| REF 14 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020218) | |||||
| REF 15 | ClinicalTrials.gov (NCT00062036) Cyclophosphamide and Fludarabine Followed By Interleukin-2 Gene-Modified Tumor Infiltrating Lymphocytes in Treating Patients With Metastatic Melanoma. U.S. National Institutes of Health. | |||||
| REF 16 | Pharmacokinetics (PK) and immunologic responses in a phase I/II study of a sustained release formulation of IL-2 in renal cell carcinoma (RCC) patients. J Clin Oncol (Meeting Abstracts) June 2006 vol. 24 no. 18_suppl 2558. | |||||
| REF 17 | ClinicalTrials.gov (NCT03954067) A Study of an Intratumoral Oncolytic Virus in Patients With Advanced Metastatic Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 18 | ClinicalTrials.gov (NCT04009681) A Study Evaluating Safety and Therapeutic Activity of THOR-707 in Adult Subjects With Advanced or Metastatic Solid Tumors (THOR-707-101). U.S. National Institutes of Health. | |||||
| REF 19 | ClinicalTrials.gov (NCT00223899) A Trial to Evaluate the Safety of Intratumoral VCL-IM01 Followed by Electroporation in Metastatic Melanoma. U.S. National Institutes of Health. | |||||
| REF 20 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800027551) | |||||
| REF 21 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800019789) | |||||
| REF 22 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800004906) | |||||
| REF 23 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008879) | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800001478) | |||||
| REF 25 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800009282) | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800023655) | |||||
| REF 27 | The AIR inhaled insulin system: system components and pharmacokinetic/glucodynamic data. Diabetes Technol Ther. 2007 Jun;9 Suppl 1:S41-7. | |||||
| REF 28 | Clinical pipeline report, company report or official report of APEIRON Biologics. | |||||
| REF 29 | Clinical pipeline report, company report or official report of Bioniz Therapeutics. | |||||
| REF 30 | J Clin Oncol 32:5s, 2014 (suppl; abstr 2071). | |||||
| REF 31 | Clinical pipeline report, company report or official report of Immunservice. | |||||
| REF 32 | Immunotherapy of high-risk acute leukemia with a recipient (autologous) vaccine expressing transgenic human CD40L and IL-2 after chemotherapy and a... Blood. 2006 Feb 15;107(4):1332-41. | |||||
| REF 33 | National Cancer Institute Drug Dictionary (drug id 665656). | |||||
| REF 34 | Intratumoral interleukin 2 for renal-cell carcinoma by direct gene transfer of a plasmid DNA/DMRIE/DOPE lipid complex. World J Urol. 2000 Apr;18(2):152-6. | |||||
| REF 35 | Evaluation of efficacy and safety of thymus humoral factor-gamma 2 in the management of chronic hepatitis B. J Hepatol. 1995 Jul;23(1):21-7. | |||||
| REF 36 | Genetic modification of human T lymphocytes for the treatment of hematologic malignancies. Haematologica. 2012 November; 97(11): 1622-1631. | |||||
| REF 37 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 38 | Clinical pipeline report, company report or official report of Astellas Pharma. | |||||
| REF 39 | Clinical pipeline report, company report or official report of Sanofi. | |||||
| REF 40 | Interleukin-2 gene therapy in a patient with glioblastoma. Gene Ther. 1995 Mar;2(2):164-7. | |||||
| REF 41 | Expression, purification and characterization of recombinant human interleukin-2-serum albumin (rhIL-2-HSA) fusion protein in Pichia pastoris. Protein Expr Purif. 2012 Jul;84(1):154-60. | |||||
| REF 42 | Development of a botanical anti-arthritis drug, PMI-001. SBIR.STTR America's Seed Fund. | |||||
| REF 43 | WO patent application no. 2013,1850,32, Nanotherapeutics for drug targeting. | |||||
| REF 44 | CN patent application no. 101039956, Cell surface glycoprotein. | |||||
| REF 45 | The novel immunomodulator, Linomide, stimulates interleukin-2-induced human natural killer (NK) cell and PHA-stimulated T cell proliferation from normal donors. Leuk Res. 1996 Jan;20(1):57-63. | |||||
| REF 46 | Melanoma and Immunotherapy. Hematology/Oncology Clinics of North America Volume 23, Issue 3, June 2009, Pages 547-564. | |||||
| REF 47 | BioPartnering North America--Programs from Pharma in Europe and the Middle East. IDrugs. 2010 Mar;13(3):162-5. | |||||
| REF 48 | An anti-IL-2 antibody increases serum half-life and improves anti-tumor efficacy of human recombinant interleukin-2. Immunopharmacology. 1994 Nov-Dec;28(3):223-32. | |||||
| REF 49 | Randomized study of recombinant interleukin-2 after autologous bone marrow transplantation for acute leukemia in first complete remission. Eur Cytokine Netw. 2000 Mar;11(1):91-8. | |||||
| REF 50 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 51 | Technology evaluation: TG-1031, Transgene SA.Curr Opin Mol Ther.2000 Feb;2(1):106-11. | |||||
| REF 52 | Immunotherapy of metastatic melanoma by intratumoral injections of Vero cells producing human IL-2: phase II randomized study comparing two dose le... Cancer Gene Ther. 2002 Mar;9(3):289-95. | |||||
| REF 53 | Potent small-molecule binding to a dynamic hot spot on IL-2. J Am Chem Soc. 2003 Dec 17;125(50):15280-1. | |||||
| REF 54 | Hot-spot mimicry of a cytokine receptor by a small molecule. Proc Natl Acad Sci U S A. 2006 Oct 17;103(42):15422-7. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

