Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T70067
(Former ID: TTDC00266)
|
|||||
| Target Name |
TRAIL receptor 2 (TRAIL-R2)
|
|||||
| Synonyms |
ZTNFR9; UNQ160/PRO186; Tumor necrosis factor receptor superfamily member 10B; TRICK2; TRAILR2; TNF-related apoptosis-inducing ligand receptor 2; KILLER; Death receptor 5; DR5; CD262
Click to Show/Hide
|
|||||
| Gene Name |
TNFRSF10B
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 4 Target-related Diseases | + | ||||
| 1 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| 2 | Liver cancer [ICD-11: 2C12] | |||||
| 3 | Colorectal cancer [ICD-11: 2B91] | |||||
| 4 | Malignant haematopoietic neoplasm [ICD-11: 2B33] | |||||
| Function |
The adapter molecule FADD recruits caspase-8 to the activated receptor. The resulting death-inducing signaling complex (DISC) performs caspase-8 proteolytic activation which initiates the subsequent cascade of caspases (aspartate-specific cysteine proteases) mediating apoptosis. Promotes the activation of NF-kappa-B. Essential for ER stress-induced apoptosis. Receptor for the cytotoxic ligand TNFSF10/TRAIL.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MEQRGQNAPAASGARKRHGPGPREARGARPGPRVPKTLVLVVAAVLLLVSAESALITQQD
LAPQQRAAPQQKRSSPSEGLCPPGHHISEDGRDCISCKYGQDYSTHWNDLLFCLRCTRCD SGEVELSPCTTTRNTVCQCEEGTFREEDSPEMCRKCRTGCPRGMVKVGDCTPWSDIECVH KESGTKHSGEVPAVEETVTSSPGTPASPCSLSGIIIGVTVAAVVLIVAVFVCKSLLWKKV LPYLKGICSGGGGDPERVDRSSQRPGAEDNVLNEIVSILQPTQVPEQEMEVQEPAEPTGV NMLSPGESEHLLEPAEAERSQRRRLLVPANEGDPTETLRQCFDDFADLVPFDSWEPLMRK LGLMDNEIKVAKAEAAGHRDTLYTMLIKWVNKTGRDASVHTLLDALETLGERLAKQKIED HLLSSGKFMYLEGNADSAMS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| ADReCS ID | BADD_A06535 | |||||
| HIT2.0 ID | T30L86 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 8 Clinical Trial Drugs | + | ||||
| 1 | Conatumumab | Drug Info | Phase 2 | Colorectal cancer | [2] | |
| 2 | Lexatumumab | Drug Info | Phase 2 | Solid tumour/cancer | [3] | |
| 3 | Anti-DR5 cells | Drug Info | Phase 1/2 | Hepatocellular carcinoma | [4] | |
| 4 | BI 905711 | Drug Info | Phase 1 | Solid tumour/cancer | [5] | |
| 5 | DS-8273 | Drug Info | Phase 1 | Solid tumour/cancer | [6] | |
| 6 | GEN1029 | Drug Info | Phase 1 | Solid tumour/cancer | [7] | |
| 7 | IGM-8444 | Drug Info | Phase 1 | Solid tumour/cancer | [8] | |
| 8 | INBRX-109 | Drug Info | Phase 1 | Solid tumour/cancer | [9] | |
| Discontinued Drug(s) | [+] 3 Discontinued Drugs | + | ||||
| 1 | RhApo2L/TRAIL | Drug Info | Discontinued in Phase 1/2 | Solid tumour/cancer | [10] | |
| 2 | HGS-TR2J | Drug Info | Discontinued in Phase 1 | Solid tumour/cancer | [11] | |
| 3 | LBY-135 | Drug Info | Discontinued in Phase 1 | Solid tumour/cancer | [12] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Agonist | [+] 5 Agonist drugs | + | ||||
| 1 | Conatumumab | Drug Info | [1], [13] | |||
| 2 | DS-8273 | Drug Info | [16] | |||
| 3 | GEN1029 | Drug Info | [17] | |||
| 4 | IGM-8444 | Drug Info | [18] | |||
| 5 | RhApo2L/TRAIL | Drug Info | [1], [20] | |||
| CAR-T-Cell-Therapy | [+] 1 CAR-T-Cell-Therapy drugs | + | ||||
| 1 | Anti-DR5 cells | Drug Info | [4] | |||
| Activator | [+] 1 Activator drugs | + | ||||
| 1 | BI 905711 | Drug Info | [15] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Viral protein interaction with cytokine and cytokine receptor | hsa04061 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| p53 signaling pathway | hsa04115 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Apoptosis | hsa04210 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
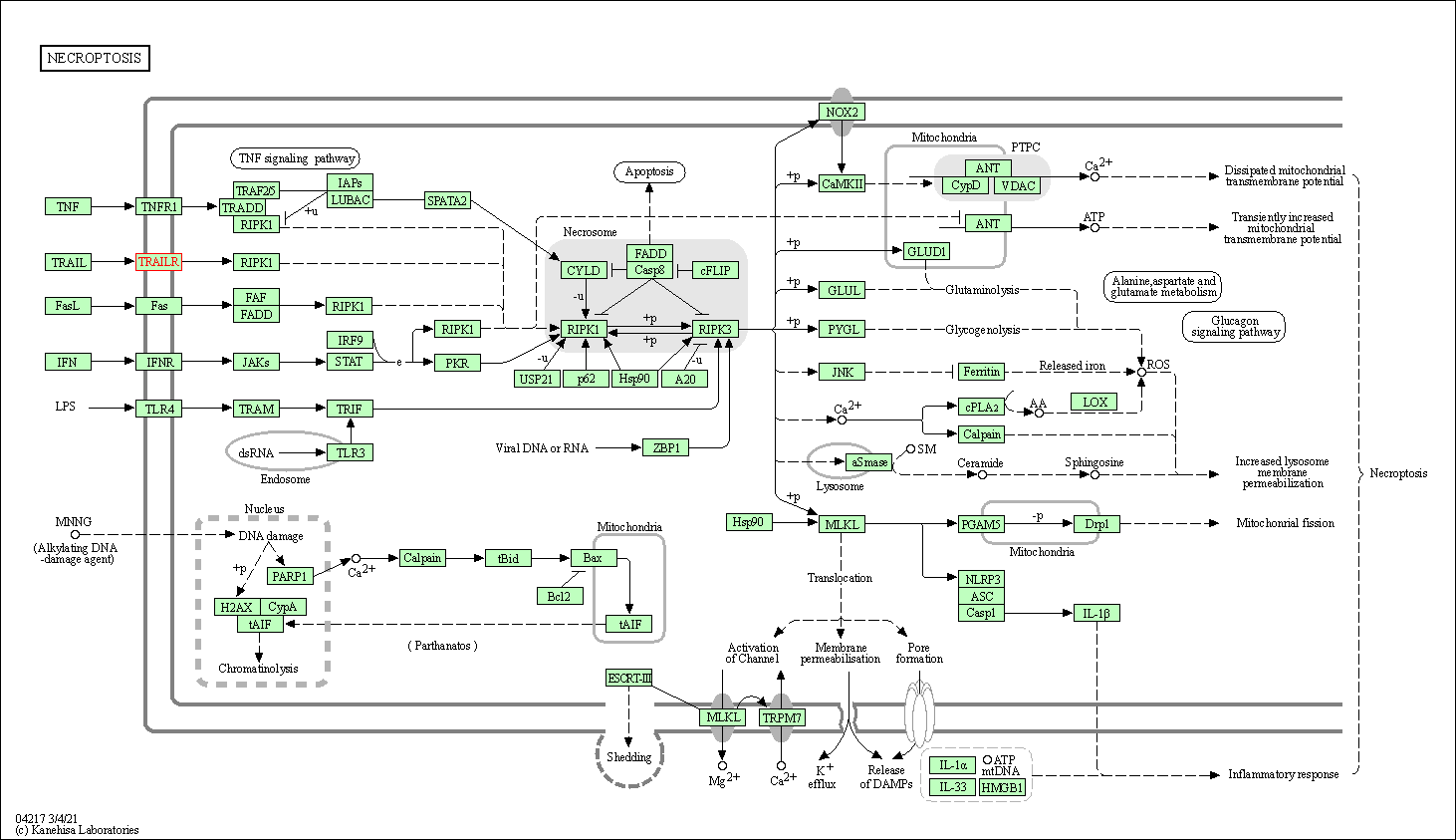
| Necroptosis | hsa04217 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
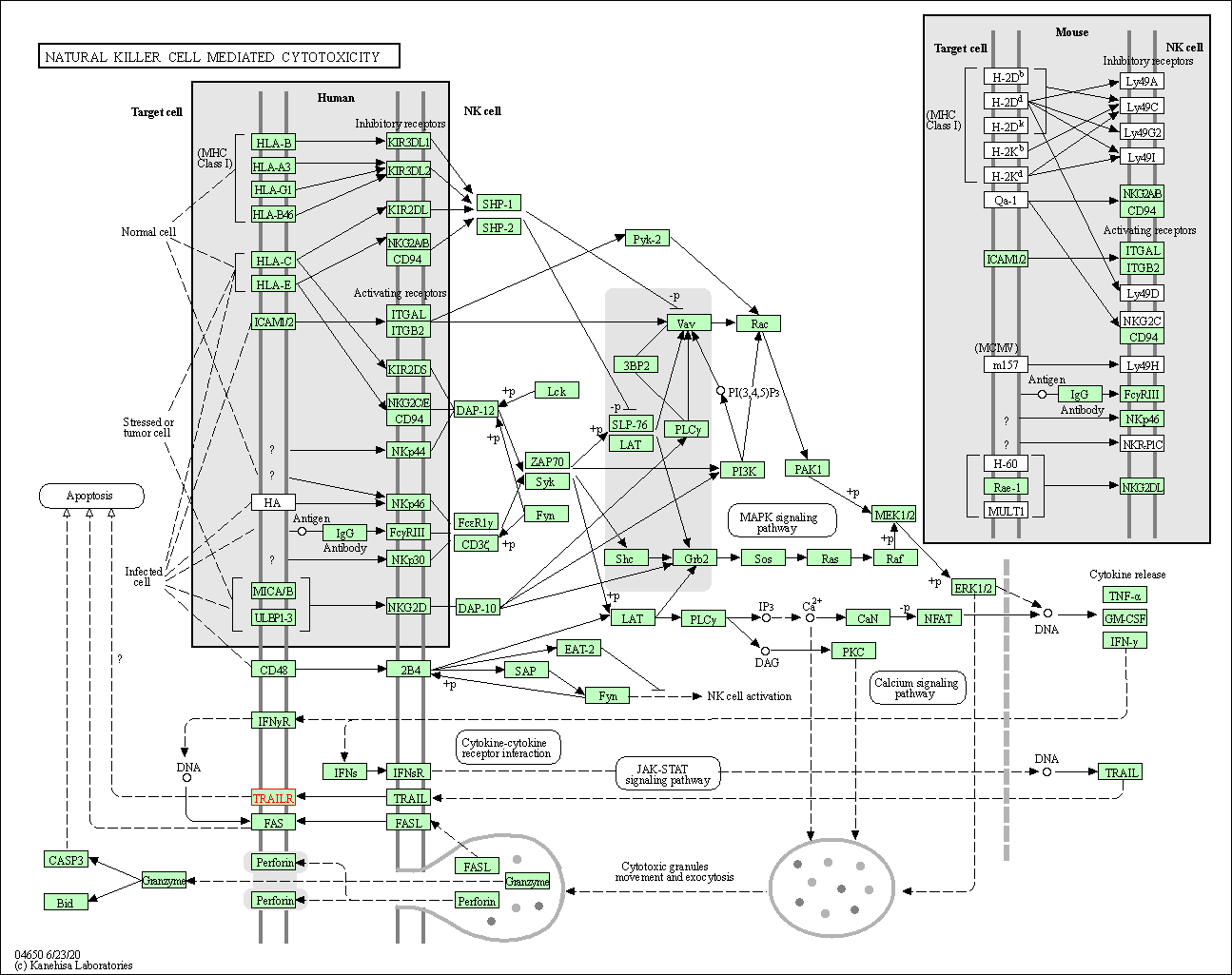
| Natural killer cell mediated cytotoxicity | hsa04650 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 12 | Degree centrality | 1.29E-03 | Betweenness centrality | 5.22E-05 |
|---|---|---|---|---|---|
| Closeness centrality | 2.38E-01 | Radiality | 1.42E+01 | Clustering coefficient | 7.42E-01 |
| Neighborhood connectivity | 5.30E+01 | Topological coefficient | 1.36E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of Amgen (2009). | |||||
| REF 2 | ClinicalTrials.gov (NCT01327612) Open Label Extension Study of Conatumumab and AMG 479. U.S. National Institutes of Health. | |||||
| REF 3 | Drug evaluation: lexatumumab, an intravenous human agonistic mAb targeting TRAIL receptor 2. Curr Opin Mol Ther. 2006 Dec;8(6):539-46. | |||||
| REF 4 | ClinicalTrials.gov (NCT03638206) Autologous CAR-T/TCR-T Cell Immunotherapy for Malignancies | |||||
| REF 5 | ClinicalTrials.gov (NCT04137289) A Study to Find a Safe and Effective Dose of BI 905711 in Patients With Advanced Gastrointestinal Cancer. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT02076451) Open-label Study of DS-8273a to Assess Its Safety and Tolerability, and Assess Its Pharmacokinetic and Pharmacodynamic Properties in Subjects With Advanced Solid Tumors or Lymphomas. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT03576131) GEN1029 (HexaBody-DR5/DR5) Safety Trial in Patients With Malignant Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT04553692) Study of IGM-8444 as a Single Agent and in Combination With Chemotherapy-based Regimens in Subjects With Solid Cancers. U.S. National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT03715933) Phase 1 Study of INBRX-109 in Subjects With Locally Advanced or Metastatic Solid Tumors Including Sarcomas. U.S. National Institutes of Health. | |||||
| REF 10 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800011860) | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021611) | |||||
| REF 12 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026275) | |||||
| REF 13 | ClinicalTrials.gov (NCT00534027) Amgen. Report of Amgen. January 22, 2009. | |||||
| REF 14 | National Cancer Institute Drug Dictionary (drug id 528015). | |||||
| REF 15 | Selective Tumor Cell Apoptosis and Tumor Regression in CDH17-Positive Colorectal Cancer Models using BI 905711, a Novel Liver-Sparing TRAILR2 Agonist. Mol Cancer Ther. 2021 Jan;20(1):96-108. | |||||
| REF 16 | First-in-human study of the antibody DR5 agonist DS-8273a in patients with advanced solid tumors. Invest New Drugs. 2017 Jun;35(3):298-306. | |||||
| REF 17 | National Cancer Institute Drug Dictionary (drug name Tilogotamab). | |||||
| REF 18 | Clinical pipeline report, company report or official report of IGM Biosciences. | |||||
| REF 19 | Clinical pipeline report, company report or official report of Inhibrx. | |||||
| REF 20 | ClinicalTrials.gov (NCT00508625) Amgen. Report of Amgen. July 2007. | |||||
| REF 21 | Enhancement of Glioma Radiation Therapy and Chemotherapy Response with Targeted Antibody Therapy Against Death Receptor 5. Int J Radiat Oncol Biol Phys. 2008 June 1; 71(2): 507-516. | |||||
| REF 22 | Safety, pharmacokinetics, and pharmacodynamics of the DR5 antibody LBY135 alone and in combination with capecitabine in patients with advanced solid tumors. Invest New Drugs. 2014 Feb;32(1):135-44. | |||||
| REF 23 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1880). | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

