Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T00176
(Former ID: TTDC00206)
|
|||||
| Target Name |
Ubiquitin-protein ligase E3 Mdm2 (MDM2)
|
|||||
| Synonyms |
RING-type E3 ubiquitin transferase Mdm2; P53-binding protein Mdm2; Oncoprotein Mdm2; MDM2 protein; Hdm2; E3 ubiquitin-protein ligase Mdm2; Double minute 2 protein
Click to Show/Hide
|
|||||
| Gene Name |
MDM2
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Acute myeloid leukaemia [ICD-11: 2A60] | |||||
| 2 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| Function |
Inhibits p53/TP53- and p73/TP73-mediated cell cycle arrest and apoptosis by binding its transcriptional activation domain. Also acts as a ubiquitin ligase E3 toward itself and ARRB1. Permits the nuclear export of p53/TP53. Promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma RB1 protein. Inhibits DAXX-mediated apoptosis by inducing its ubiquitination and degradation. Component of the TRIM28/KAP1-MDM2-p53/TP53 complex involved in stabilizing p53/TP53. Also component of the TRIM28/KAP1-ERBB4-MDM2 complex which links growth factor and DNA damage response pathways. Mediates ubiquitination and subsequent proteasome degradation of DYRK2 in nucleus. Ubiquitinates IGF1R and SNAI1 and promotes them to proteasomal degradation. Ubiquitinates DCX, leading to DCX degradation and reduction of the dendritic spine density of olfactory bulb granule cells. Ubiquitinates DLG4, leading to proteasomal degradation of DLG4 which is required for AMPA receptor endocytosis. E3 ubiquitin-protein ligase that mediates ubiquitination of p53/TP53, leading to its degradation by the proteasome.
Click to Show/Hide
|
|||||
| BioChemical Class |
Acyltransferase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.3.2.27
|
|||||
| Sequence |
MCNTNMSVPTDGAVTTSQIPASEQETLVRPKPLLLKLLKSVGAQKDTYTMKEVLFYLGQY
IMTKRLYDEKQQHIVYCSNDLLGDLFGVPSFSVKEHRKIYTMIYRNLVVVNQQESSDSGT SVSENRCHLEGGSDQKDLVQELQEEKPSSSHLVSRPSTSSRRRAISETEENSDELSGERQ RKRHKSDSISLSFDESLALCVIREICCERSSSSESTGTPSNPDLDAGVSEHSGDWLDQDS VSDQFSVEFEVESLDSEDYSLSEEGQELSDEDDEVYQVTVYQAGESDTDSFEEDPEISLA DYWKCTSCNEMNPPLPSHCNRCWALRENWLPEDKGKDKGEISEKAKLENSTQAEEGFDVP DCKKTIVNDSRESCVEENDDKITQASQSQESEDYSQPSTSSSIIYSSQEDVKEFEREETQ DKEESVESSLPLNAIEPCVICQGRPKNGCIVHGKTGHLMACFTCAKKLKKRNKPCPVCRQ PIQMIVLTYFP Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T06PVX | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 10 Clinical Trial Drugs | + | ||||
| 1 | RG7388 | Drug Info | Phase 3 | Solid tumour/cancer | [2] | |
| 2 | ALRN-6924 | Drug Info | Phase 2 | Haematological malignancy | [3] | |
| 3 | AMG 232 | Drug Info | Phase 2 | Merkel cell carcinoma | [4] | |
| 4 | APG-115 | Drug Info | Phase 2 | Prolymphocytic leukaemia | [5] | |
| 5 | ASTX295 | Drug Info | Phase 1/2 | Solid tumour/cancer | [6] | |
| 6 | BI 907828 | Drug Info | Phase 1 | Solid tumour/cancer | [7] | |
| 7 | DS-3032 | Drug Info | Phase 1 | Solid tumour/cancer | [8] | |
| 8 | HDM201 | Drug Info | Phase 1 | Haematological malignancy | [9] | |
| 9 | JNJ-26854165 | Drug Info | Phase 1 | Prostate cancer | [10] | |
| 10 | RG7775 | Drug Info | Phase 1 | Acute myeloid leukaemia | [11] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Modulator | [+] 3 Modulator drugs | + | ||||
| 1 | RG7388 | Drug Info | [1] | |||
| 2 | AMG 232 | Drug Info | [13] | |||
| 3 | JNJ-26854165 | Drug Info | [17] | |||
| Antagonist | [+] 2 Antagonist drugs | + | ||||
| 1 | ALRN-6924 | Drug Info | [3], [12] | |||
| 2 | ASTX295 | Drug Info | [14] | |||
| Inhibitor | [+] 6 Inhibitor drugs | + | ||||
| 1 | APG-115 | Drug Info | [3] | |||
| 2 | BI 907828 | Drug Info | [15] | |||
| 3 | DS-3032 | Drug Info | [16] | |||
| 4 | HDM201 | Drug Info | [3] | |||
| 5 | RG7775 | Drug Info | [18] | |||
| 6 | MI-219 | Drug Info | [19] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: SAR-405838 | Ligand Info | |||||
| Structure Description | MDM2 in complex with SAR405838 | PDB:5TRF | ||||
| Method | X-ray diffraction | Resolution | 2.10 Å | Mutation | No | [20] |
| PDB Sequence |
TDGAVTTSQI
19 PASEQETLVR29 PKPLLLKLLK39 SVGAQKDTYT49 MKEVLFYLGQ59 YIMTKRLYQH 73 IVYCSNDLLG83 DLFGVPSFSV93 KEHRKIYTMI103 YRNLVVVN
|
|||||
|
|
||||||
| Ligand Name: HDM201 | Ligand Info | |||||
| Structure Description | HDM2 (17-111, WILD TYPE) COMPLEXED WITH NVP-HDM201 AT 1.56A | PDB:5OC8 | ||||
| Method | X-ray diffraction | Resolution | 1.56 Å | Mutation | No | [21] |
| PDB Sequence |
IPASEQETLV
28 RPKPLLLKLL38 KSVGAQKDTY48 TMKEVLFYLG58 QYIMTKRLYD68 EKQQHIVYCS 78 NDLLGDLFGV88 PSFSVKEHRK98 IYTMIYRNLV108 VVN
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|



| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| FoxO signaling pathway | hsa04068 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Cell cycle | hsa04110 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
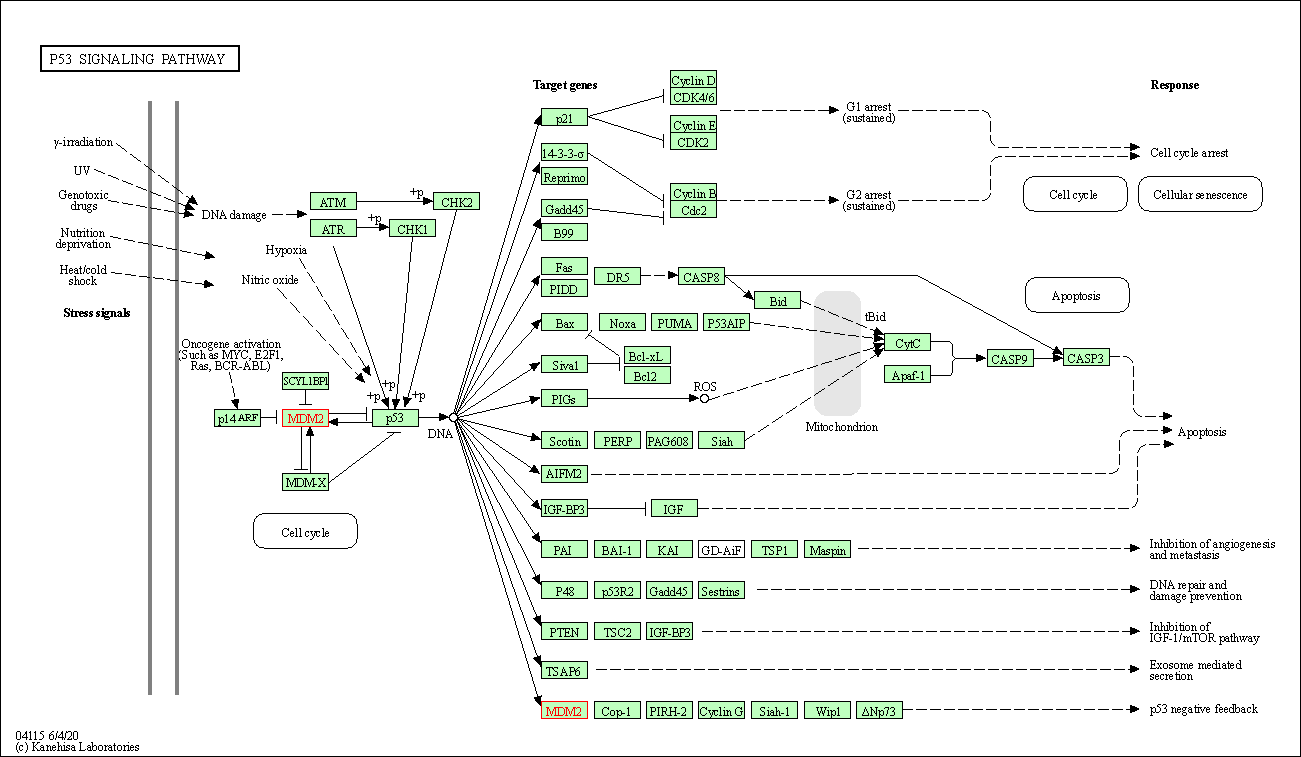
| p53 signaling pathway | hsa04115 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
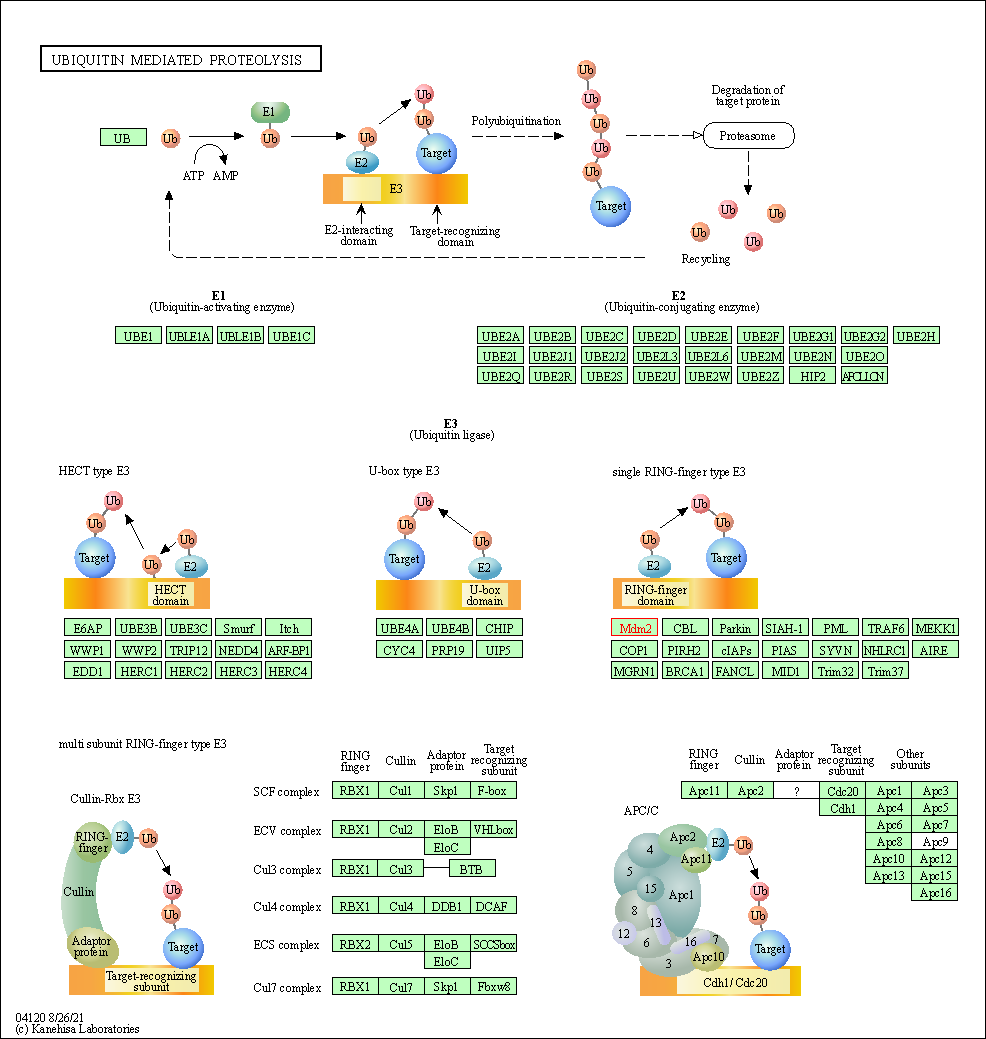
| Ubiquitin mediated proteolysis | hsa04120 | Affiliated Target |

|
| Class: Genetic Information Processing => Folding, sorting and degradation | Pathway Hierarchy | ||
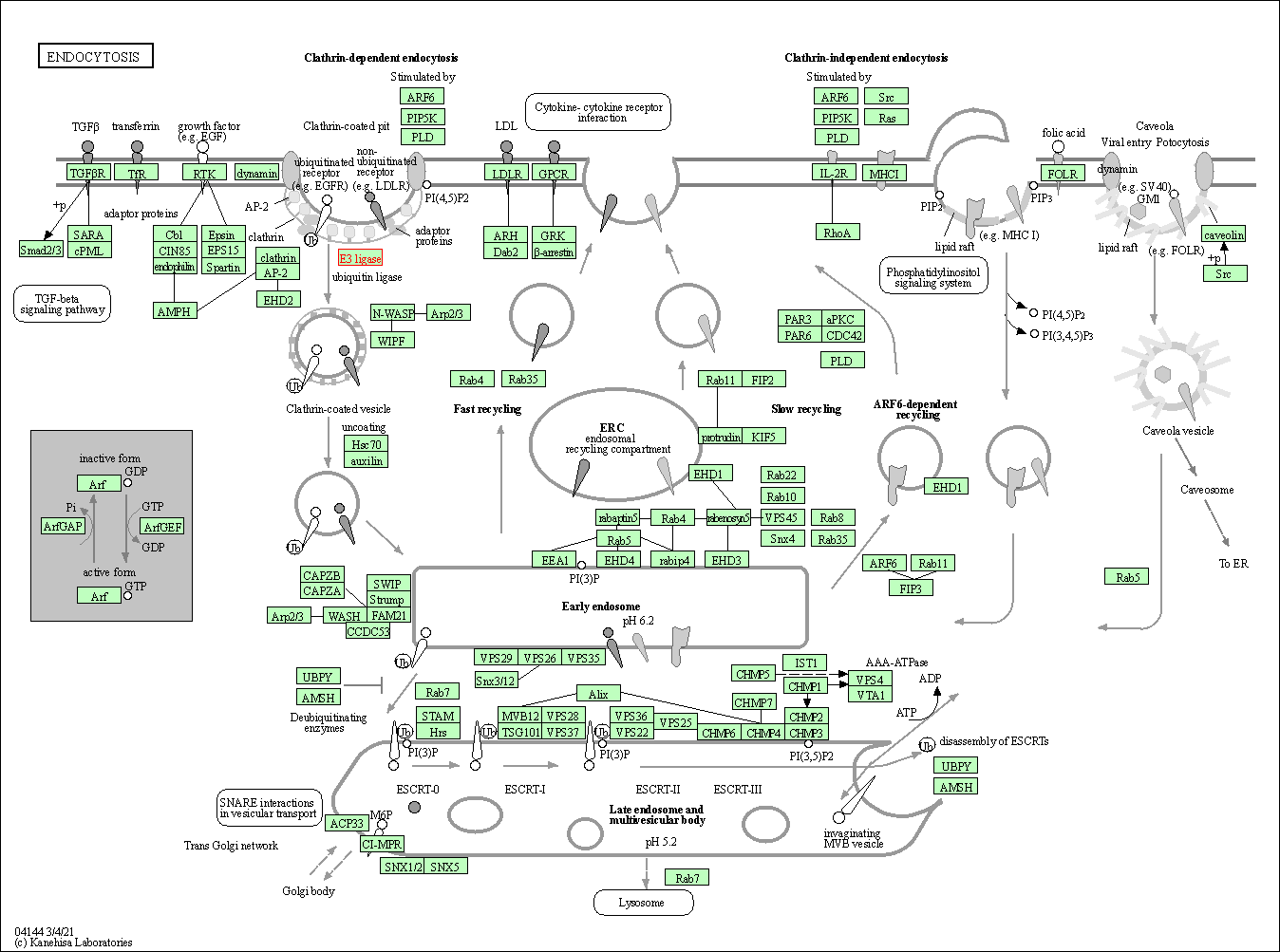
| Endocytosis | hsa04144 | Affiliated Target |

|
| Class: Cellular Processes => Transport and catabolism | Pathway Hierarchy | ||
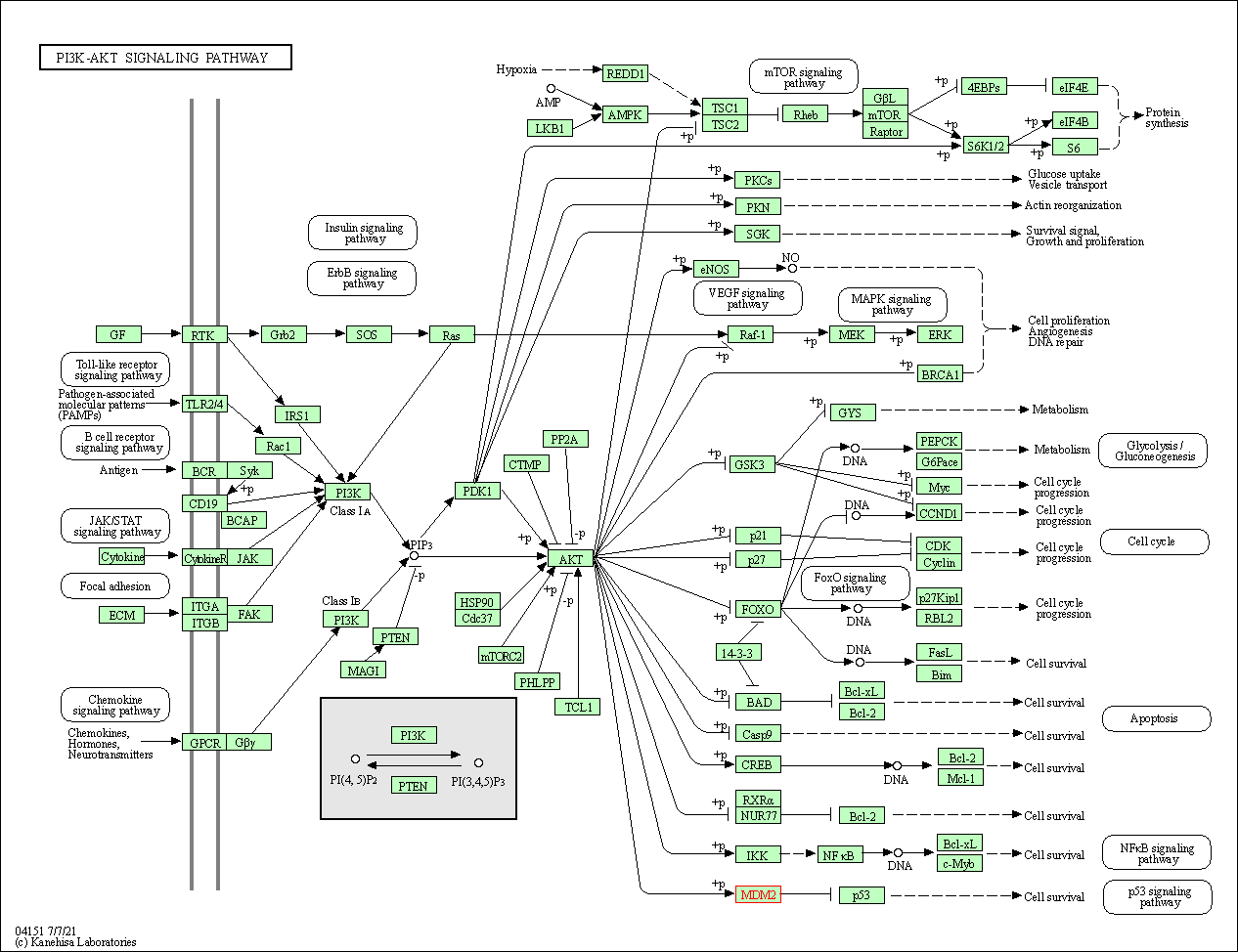
| PI3K-Akt signaling pathway | hsa04151 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Cellular senescence | hsa04218 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
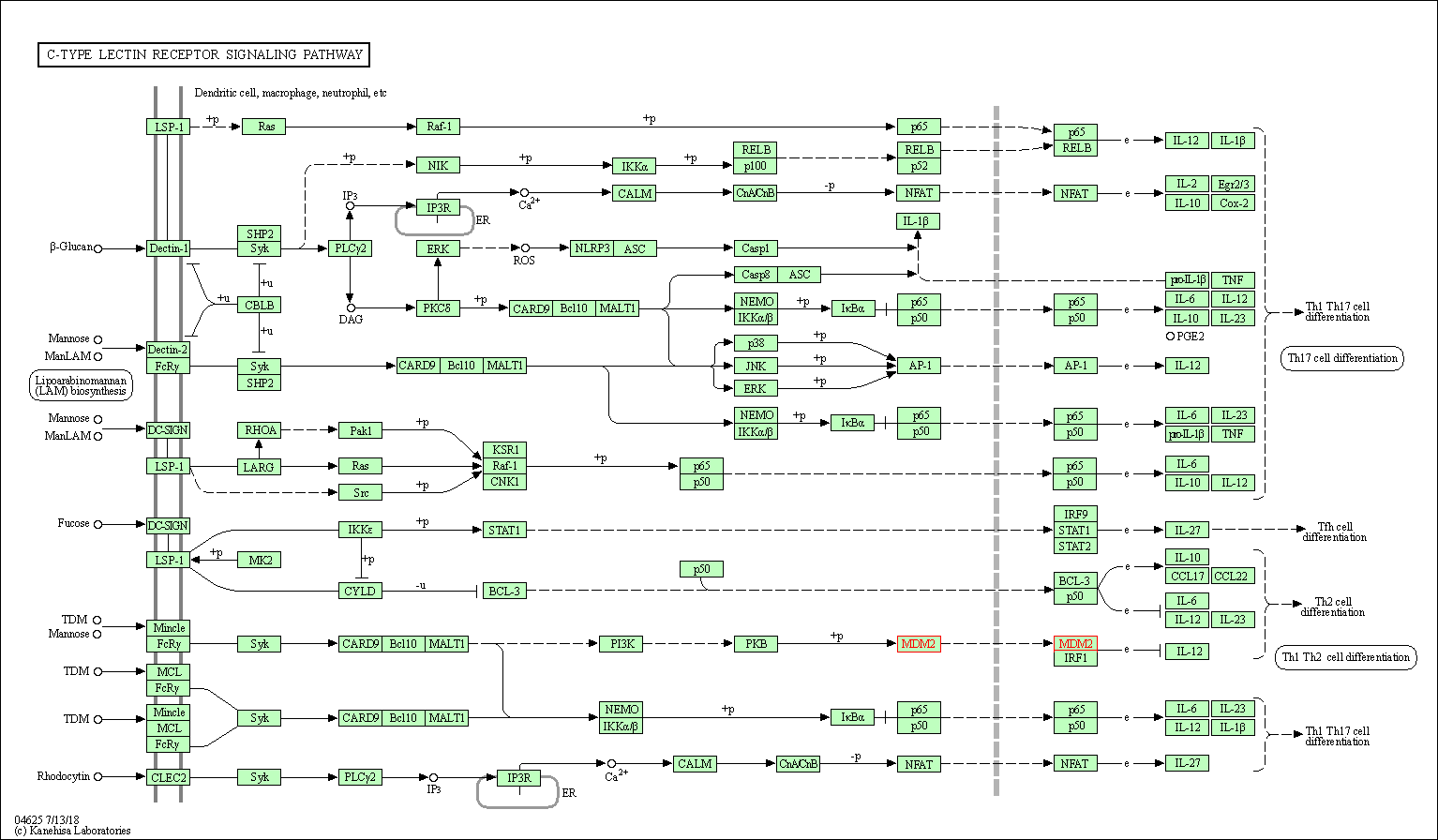
| C-type lectin receptor signaling pathway | hsa04625 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
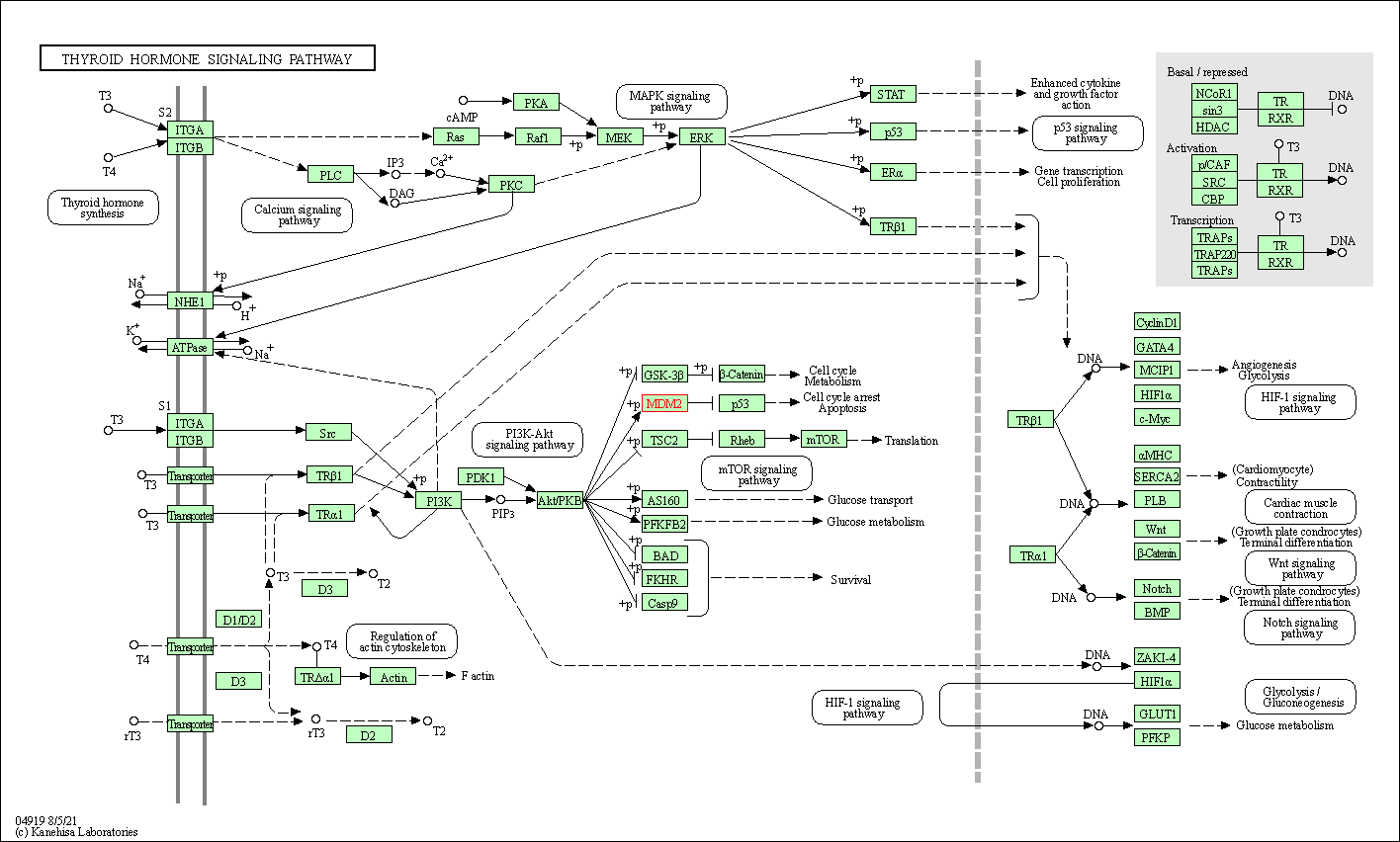
| Thyroid hormone signaling pathway | hsa04919 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 74 | Degree centrality | 7.95E-03 | Betweenness centrality | 1.32E-02 |
|---|---|---|---|---|---|
| Closeness centrality | 2.78E-01 | Radiality | 1.47E+01 | Clustering coefficient | 1.02E-01 |
| Neighborhood connectivity | 6.00E+01 | Topological coefficient | 3.56E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Pre-clinical evaluation of the MDM2-p53 antagonist RG7388 alone and in combination with chemotherapy in neuroblastoma. Oncotarget. 2015 Apr 30;6(12):10207-21. | |||||
| REF 2 | Small molecules, big targets: drug discovery faces the protein-protein interaction challenge.Nat Rev Drug Discov. 2016 Aug;15(8):533-50. | |||||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 4 | ClinicalTrials.gov (NCT03787602) This Study Evaluates KRT-232, a Novel Oral Small Molecule Inhibitor of MDM2, for the Treatment of Patients With (p53WT) Merkel Cell Carcinoma Who Have Failed Anti-PD-1/ PD-L1 Immunotherapy. U.S. National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT04496349) A Study Evaluating APG-115 as a Single Agent or in Combination With APG-2575 in Subjects With T-PLL. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT03975387) Study of ASTX295 in Patients With Solid Tumors With Wild-Type p53. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT03449381) This Study Aims to Find the Best Dose of BI 907828 in Patients With Different Types of Advanced Cancer (Solid Tumors). U.S. National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT01877382) A Phase 1 Multiple Ascending Dose Study of DS-3032b, an Oral Murine Double Minute 2 (MDM2) Inhibitor, in Subjects With Advanced Solid Tumors or Lymphomas. U.S. National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT02143635) Study to Determine and Evaluate a Safe and Tolerated Dose of HDM201 in Patients With Selected Advanced Tumors That Are TP53wt. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT00676910) A Research Study of JNJ-26854165 to Determine the Safety and Dose in Patients With Advanced Stage or Refractory Solid Tumors.. U.S. National Institutes of Health. | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800040360) | |||||
| REF 12 | Anti-ageing pipeline starts to mature.Nat Rev Drug Discov. 2018 Sep;17(9):609-612. | |||||
| REF 13 | Discovery of a small molecule MDM2 inhibitor (AMG 232) for treating cancer. J Med Chem. 2014 Aug 14;57(15):6332-41. | |||||
| REF 14 | Clinical pipeline report, company report or official report of Astex Pharmaceuticals. | |||||
| REF 15 | MDM2 inhibition: an important step forward in cancer therapy. Leukemia. 2020 Nov;34(11):2858-2874. | |||||
| REF 16 | Drugging the p53 pathway: understanding the route to clinical efficacy. Nat Rev Drug Discov. 2014 Mar;13(3):217-36. | |||||
| REF 17 | Serdemetan antagonizes the Mdm2-HIF1alpha axis leading to decreased levels of glycolytic enzymes. PLoS One. 2013 Sep 6;8(9):e74741. | |||||
| REF 18 | Phase I clinical trail of RG7775 for treating Acute myelogenous leukemia. Roche. | |||||
| REF 19 | MI-219-zinc combination: a new paradigm in MDM2 inhibitor-based therapy. Oncogene. 2011 Jan 6;30(1):117-26. | |||||
| REF 20 | SAR405838: an optimized inhibitor of MDM2-p53 interaction that induces complete and durable tumor regression. Cancer Res. 2014 Oct 15;74(20):5855-65. | |||||
| REF 21 | Dose and Schedule Determine Distinct Molecular Mechanisms Underlying the Efficacy of the p53-MDM2 Inhibitor HDM201. Cancer Res. 2018 Nov 1;78(21):6257-6267. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

