Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T08499
(Former ID: TTDI03405)
|
|||||
| Target Name |
Caterpiller protein 1.1 (NLRP3)
|
|||||
| Synonyms |
PYRIN-containing APAF1-like protein 1; PYPAF1; NALP3; NACHT, LRR and PYD domains-containing protein 3; Cryopyrin; Cold-induced autoinflammatory syndrome 1 protein; CLR1.1; CIAS1; C1orf7; Angiotensin/vasopressin receptor AII/AVP-like
Click to Show/Hide
|
|||||
| Gene Name |
NLRP3
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 4 Target-related Diseases | + | ||||
| 1 | Chronic pain [ICD-11: MG30] | |||||
| 2 | Gout [ICD-11: FA25] | |||||
| 3 | Postoperative inflammation [ICD-11: 1A00-CA43] | |||||
| 4 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| Function |
In response to pathogens and other damage-associated signals, initiates the formation of the inflammasome polymeric complex, made of NLRP3, PYCARD and CASP1 (and possibly CASP4 and CASP5). Recruitment of proCASP1 to the inflammasome promotes its activation and CASP1-catalyzed IL1B and IL18 maturation and secretion in the extracellular milieu. Activation of NLRP3 inflammasome is also required for HMGB1 secretion. The active cytokines and HMGB1 stimulate inflammatory responses. Inflammasomes can also induce pyroptosis, an inflammatory form of programmed cell death. Under resting conditions, NLRP3 is autoinhibited. NLRP3 activation stimuli include extracellular ATP, reactive oxygen species, K(+) efflux, crystals of monosodium urate or cholesterol, amyloid-beta fibers, environmental or industrial particles and nanoparticles, cytosolic dsRNA, etc. However, it is unclear what constitutes the direct NLRP3 activator. Activation in presence of cytosolic dsRNA is mediated by DHX33. Independently of inflammasome activation, regulates the differentiation of T helper 2 (Th2) cells and has a role in Th2 cell-dependent asthma and tumor growth. During Th2 differentiation, required for optimal IRF4 binding to IL4 promoter and for IRF4-dependent IL4 transcription. Binds to the consensus DNA sequence 5'-GRRGGNRGAG-3'. May also participate in the transcription of IL5, IL13, GATA3, CCR3, CCR4 and MAF. As the sensor component of the NLRP3 inflammasome, plays a crucial role in innate immunity and inflammation.
Click to Show/Hide
|
|||||
| UniProt ID | ||||||
| Sequence |
MKMASTRCKLARYLEDLEDVDLKKFKMHLEDYPPQKGCIPLPRGQTEKADHVDLATLMID
FNGEEKAWAMAVWIFAAINRRDLYEKAKRDEPKWGSDNARVSNPTVICQEDSIEEEWMGL LEYLSRISICKMKKDYRKKYRKYVRSRFQCIEDRNARLGESVSLNKRYTRLRLIKEHRSQ QEREQELLAIGKTKTCESPVSPIKMELLFDPDDEHSEPVHTVVFQGAAGIGKTILARKMM LDWASGTLYQDRFDYLFYIHCREVSLVTQRSLGDLIMSCCPDPNPPIHKIVRKPSRILFL MDGFDELQGAFDEHIGPLCTDWQKAERGDILLSSLIRKKLLPEASLLITTRPVALEKLQH LLDHPRHVEILGFSEAKRKEYFFKYFSDEAQARAAFSLIQENEVLFTMCFIPLVCWIVCT GLKQQMESGKSLAQTSKTTTAVYVFFLSSLLQPRGGSQEHGLCAHLWGLCSLAADGIWNQ KILFEESDLRNHGLQKADVSAFLRMNLFQKEVDCEKFYSFIHMTFQEFFAAMYYLLEEEK EGRTNVPGSRLKLPSRDVTVLLENYGKFEKGYLIFVVRFLFGLVNQERTSYLEKKLSCKI SQQIRLELLKWIEVKAKAKKLQIQPSQLELFYCLYEMQEEDFVQRAMDYFPKIEINLSTR MDHMVSSFCIENCHRVESLSLGFLHNMPKEEEEEEKEGRHLDMVQCVLPSSSHAACSHGL VNSHLTSSFCRGLFSVLSTSQSLTELDLSDNSLGDPGMRVLCETLQHPGCNIRRLWLGRC GLSHECCFDISLVLSSNQKLVELDLSDNALGDFGIRLLCVGLKHLLCNLKKLWLVSCCLT SACCQDLASVLSTSHSLTRLYVGENALGDSGVAILCEKAKNPQCNLQKLGLVNSGLTSVC CSALSSVLSTNQNLTHLYLRGNTLGDKGIKLLCEGLLHPDCKLQVLELDNCNLTSHCCWD LSTLLTSSQSLRKLSLGNNDLGDLGVMMFCEVLKQQSCLLQNLGLSEMYFNYETKSALET LQEEKPELTVVFEPSW Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T16MRU | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 3 Clinical Trial Drugs | + | ||||
| 1 | Dapansutrile | Drug Info | Phase 2 | Acute gout flare | [1] | |
| 2 | BMS-986299 | Drug Info | Phase 1 | Solid tumour/cancer | [2] | |
| 3 | Selnoflast | Drug Info | Phase 1 | Inflammation | [3] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Inhibitor | [+] 3 Inhibitor drugs | + | ||||
| 1 | Dapansutrile | Drug Info | [1] | |||
| 2 | Selnoflast | Drug Info | [3] | |||
| 3 | MCC950 | Drug Info | [5] | |||
| Agonist | [+] 1 Agonist drugs | + | ||||
| 1 | BMS-986299 | Drug Info | [4] | |||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: MCC950 | Ligand Info | |||||
| Structure Description | Cryo-EM structure of the NLRP3 decamer bound to the inhibitor CRID3 | PDB:7PZC | ||||
| Method | Electron microscopy | Resolution | 3.90 Å | Mutation | No | [6] |
| PDB Sequence |
MASTRCKLAR
12 YLEDLEDVDL22 KKFKMHLEDY32 PPQKGCIPLP42 RGQTEKADHV52 DLATLMIDFN 62 GEEKAWAMAV72 WIFAAINRRD82 LYEKAKRDEP92 KWGSDNARVS102 NPTVICQEDS 112 IEEEWMGLLE122 YLSRISICKM132 KKDYRKKYRK142 YVRSRFQCIE152 ESVSLNKRYT 169 RLRLIKEHRS179 QSPVSPIKME206 LLFDPDDEHS216 EPVHTVVFQG226 AAGIGKTILA 236 RKMMLDWASG246 TLYQDRFDYL256 FYIHCREVSL266 VTQRSLGDLI276 MSCCPDPNPP 286 IHKIVRKPSR296 ILFLMDGFDE306 LQGAFDEHIG316 PLCTDWQKAE326 RGDILLSSLI 336 RKKLLPEASL346 LITTRPVALE356 KLQHLLDHPR366 HVEILGFSEA376 KRKEYFFKYF 386 SDEAQARAAF396 SLIQENEVLF406 TMCFIPLVCW416 IVCTGLKQQM426 ESGKSLAQTS 436 KTTTAVYVFF446 LSSLLQPRGG456 SQEHGLCAHL466 WGLCSLAADG476 IWNQKILFEE 486 SDLRNHGLQK496 ADVSAFLRMN506 LFQKEVDCEK516 FYSFIHMTFQ526 EFFAAMYYLL 536 EEEKEGRTNV546 PGSRLKLPSR556 DVTVLLENYG566 KFEKGYLIFV576 VRFLFGLVNQ 586 ERTSYLEKKL596 SCKISQQIRL606 ELLKWIEVKA616 KAKKLQIQPS626 QLELFYCLYE 636 MQEEDFVQRA646 MDYFPKIEIN656 LSTRMDHMVS666 SFCIENCHRV676 ESLSLGFLHN 686 MPKEEEEEEK696 EGRHLDMVQC706 VLPSSSHAAC716 SHGLVNSHLT726 SSFCRGLFSV 736 LSTSQSLTEL746 DLSDNSLGDP756 GMRVLCETLQ766 HPGCNIRRLW776 LGRCGLSHEC 786 CFDISLVLSS796 NQKLVELDLS806 DNALGDFGIR816 LLCVGLKHLL826 CNLKKLWLVS 836 CCLTSACCQD846 LASVLSTSHS856 LTRLYVGENA866 LGDSGVAILC876 EKAKNPQCNL 886 QKLGLVNSGL896 TSVCCSALSS906 VLSTNQNLTH916 LYLRGNTLGD926 KGIKLLCEGL 936 LHPDCKLQVL946 ELDNCNLTSH956 CCWDLSTLLT966 SSQSLRKLSL976 GNNDLGDLGV 986 MMFCEVLKQQ996 SCLLQNLGLS1006 EMYFNYETKS1016 ALETLQEEKP1026 ELTVVFEPSW 1036
|
|||||
|
|
ALA227
3.352
ALA228
2.616
GLY229
4.980
ARG351
3.460
PRO352
4.196
VAL353
4.671
MET408
3.083
ILE411
3.850
LEU413
4.787
VAL414
3.926
THR439
3.716
|
|||||
| Ligand Name: adenosine diphosphate | Ligand Info | |||||
| Structure Description | Crystal Structure of NLRP3 NACHT domain in complex with a potent inhibitor | PDB:7ALV | ||||
| Method | X-ray diffraction | Resolution | 2.83 Å | Mutation | No | [7] |
| PDB Sequence |
KDYRKKYRKY
143 VRSRFQCIEL164 NKRYTRLRLI174 KEHSPIKMEL207 LFDPDEPVHT221 VVFQGAAGIG 231 KTILARKMML241 DWASGTLYQD251 RFDYLFYIHC261 REVSLVTQRS271 LGDLIMSCCP 281 DPNPPIHKIV291 RKPSRILFLM301 DGFDELQGAF311 DEHIGPLCTD321 WQKAERGDIL 331 LSSLIRKKLL341 PEASLLITTR351 PVALEKLQHL361 LDHPRHVEIL371 GFSEAKRKEY 381 FFKYFSDEAQ391 ARAAFSLIQE401 NEVLFTMCFI411 PLVCWIVCTG421 LKQQMESGKS 431 LAQTSKTTTA441 VYVFFLSSLL451 CAHLWGLCSL472 AADGIWNQKI482 LFEESDLRNH 492 GLQDVSAFLR504 MNLFQKEVKF517 YSFIHMTFQE527 FFAAMYYLLE537 ESRDVTVLLE 563 NYGKFEKGYL573 IFVVRFLFGL583 VNQERSYLEK594 KLSCKISQQI604 RLELLKWIEV 614 KAKAKKLQIQ624 PSQLELFYCL634 YEMQEEDFVQ644 RAMDYFPKIE654 INLSTRMDHM 664 VSSFCIENCH674 RV
|
|||||
|
|
ARG167
3.366
TYR168
3.342
THR169
2.765
ARG170
4.973
LEU171
3.556
GLY226
4.909
ALA227
3.728
ALA228
3.969
GLY229
3.040
ILE230
3.761
GLY231
3.114
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
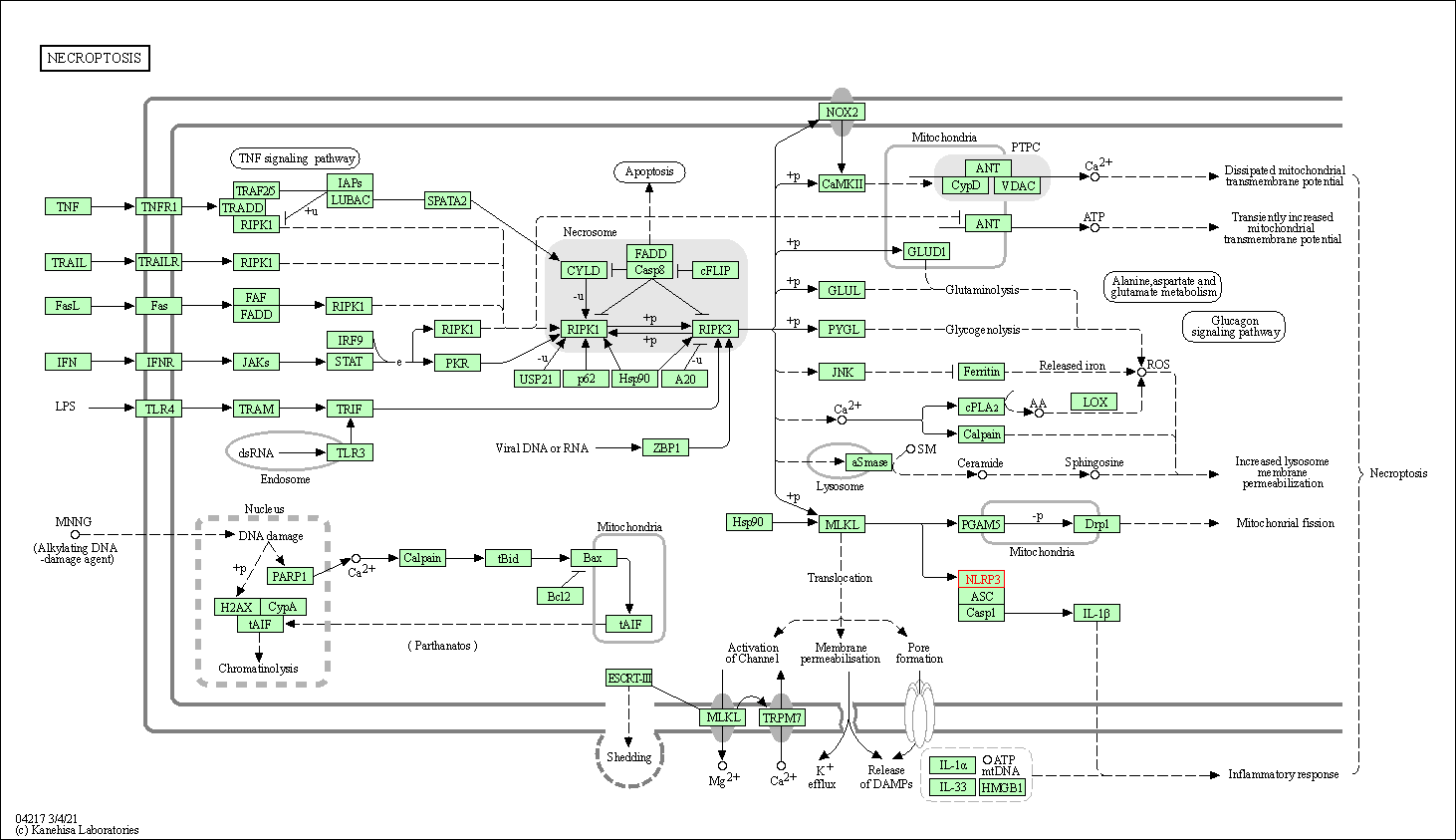
| Necroptosis | hsa04217 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
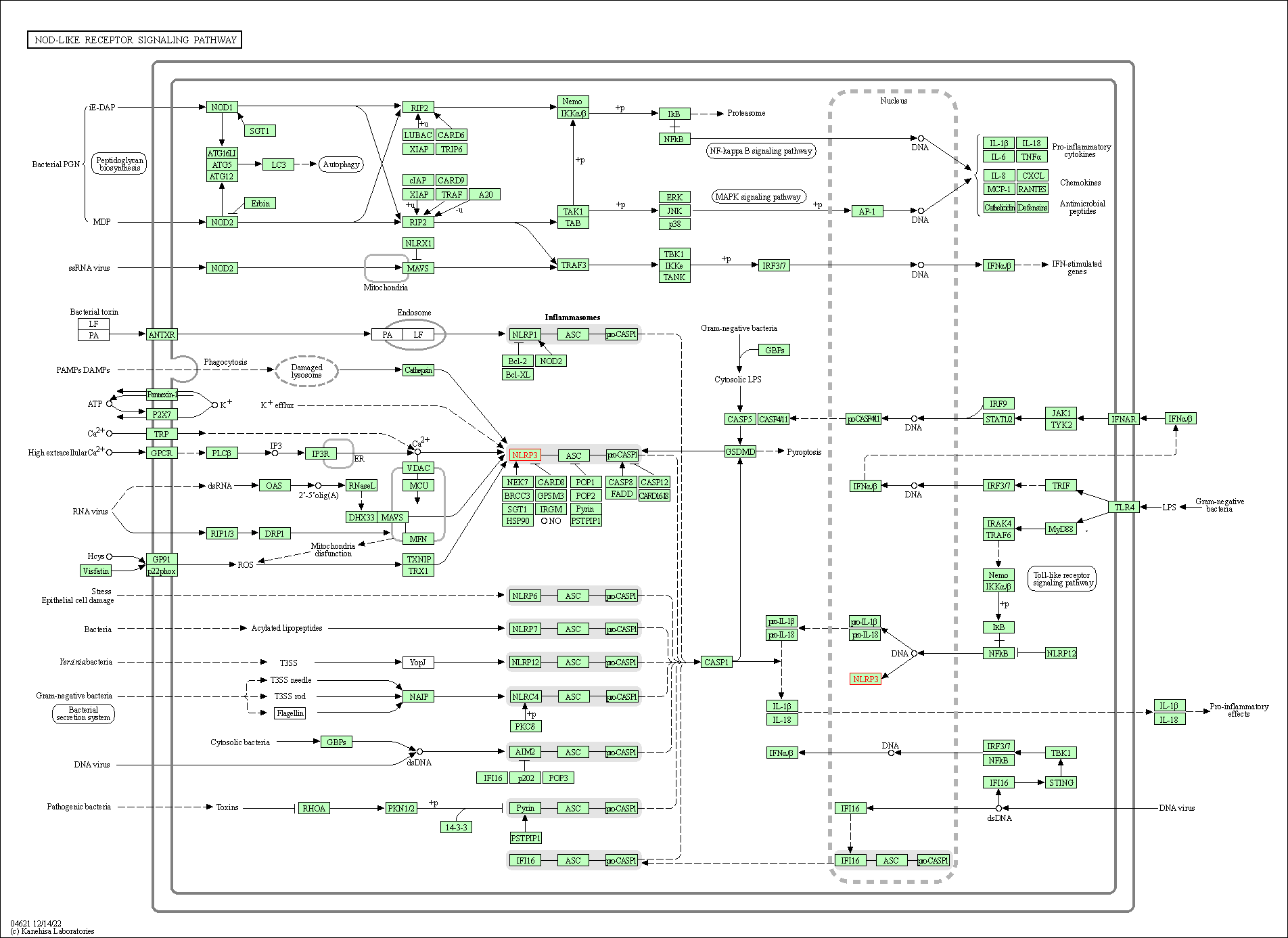
| NOD-like receptor signaling pathway | hsa04621 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
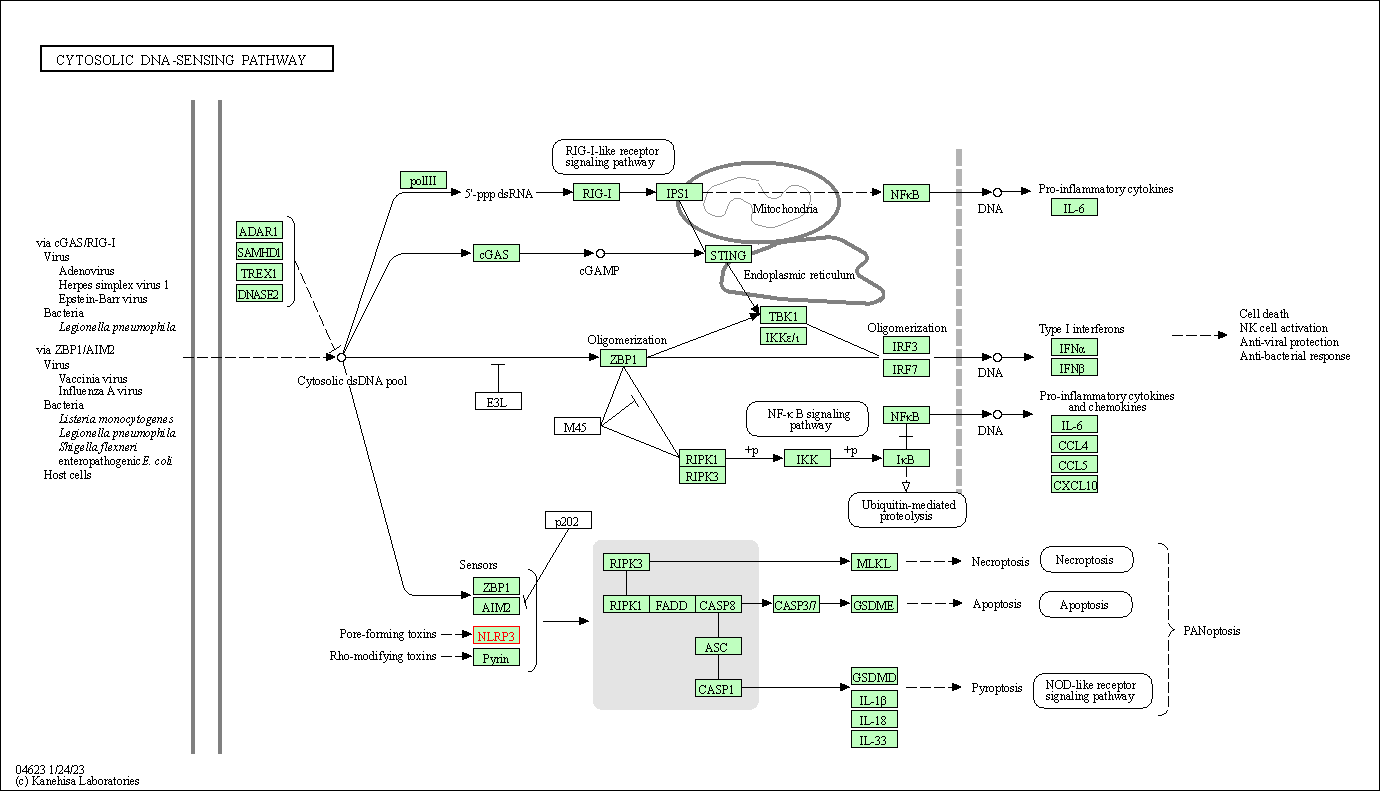
| Cytosolic DNA-sensing pathway | hsa04623 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
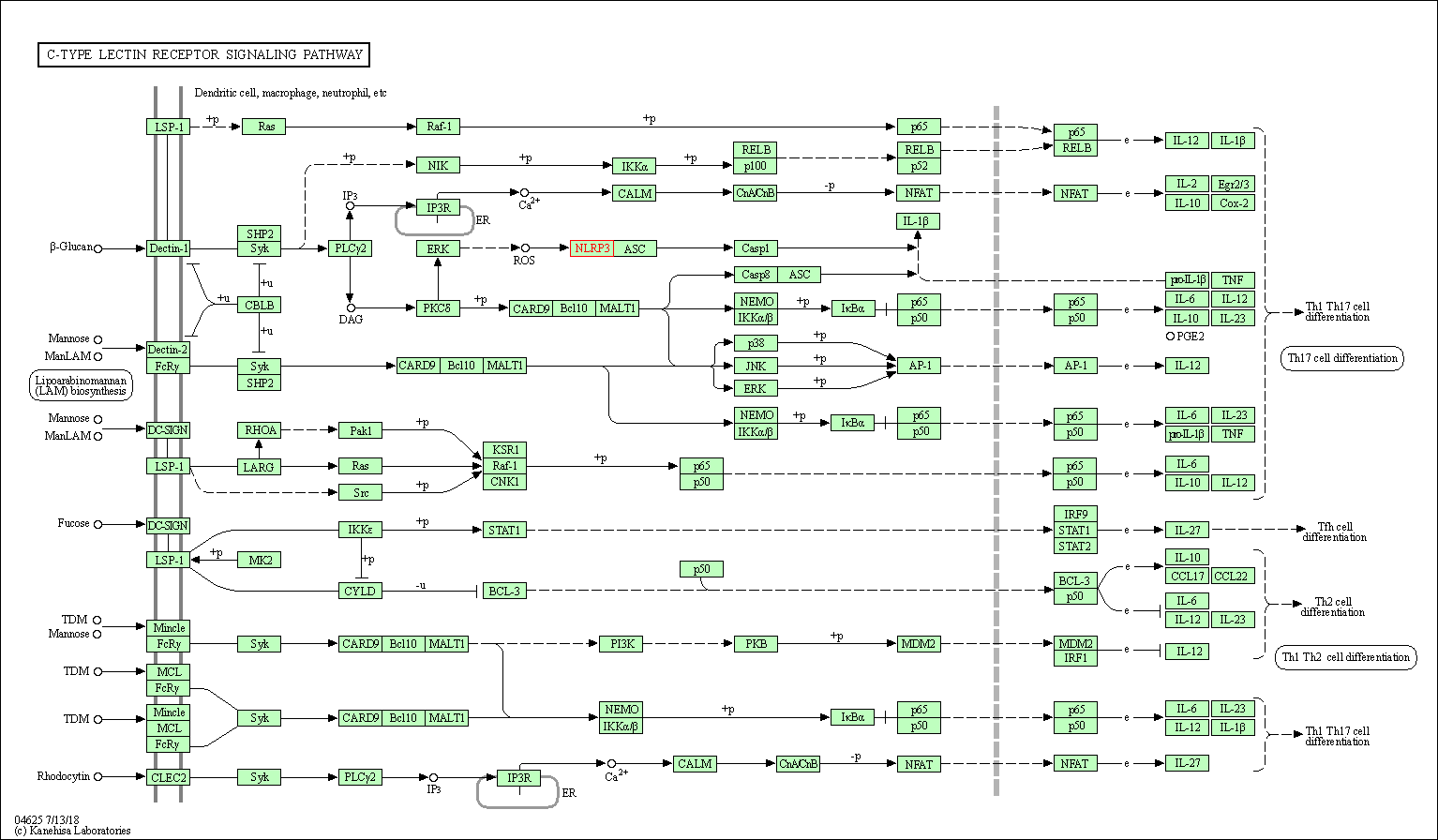
| C-type lectin receptor signaling pathway | hsa04625 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Degree | 19 | Degree centrality | 2.04E-03 | Betweenness centrality | 1.95E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.35E-01 | Radiality | 1.41E+01 | Clustering coefficient | 1.05E-01 |
| Neighborhood connectivity | 2.09E+01 | Topological coefficient | 6.51E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 2 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 3 | Clinical pipeline report, company report or official report of Roche | |||||
| REF 4 | National Cancer Institute Drug Dictionary (drug name BMS-986299). | |||||
| REF 5 | A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015 Mar;21(3):248-55. | |||||
| REF 6 | Structure of the NLRP3 decamer bound to the cytokine release inhibitor CRID3. Nature. 2022 Apr;604(7904):184-189. | |||||
| REF 7 | Crystal Structure of NLRP3 NACHT Domain With an Inhibitor Defines Mechanism of Inflammasome Inhibition. J Mol Biol. 2021 Dec 3;433(24):167309. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

