Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T10191
(Former ID: TTDC00265)
|
|||||
| Target Name |
T-cell surface glycoprotein CD4 (CD4)
|
|||||
| Synonyms |
T-cell surface antigen T4/Leu-3
Click to Show/Hide
|
|||||
| Gene Name |
CD4
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Human immunodeficiency virus disease [ICD-11: 1C60-1C62] | |||||
| Function |
In T-cells, functions primarily as a coreceptor for MHC class II molecule:peptide complex. The antigens presented by class II peptides are derived from extracellular proteins while class I peptides are derived from cytosolic proteins. Interacts simultaneously with the T-cell receptor (TCR) and the MHC class II presented by antigen presenting cells (APCs). In turn, recruits the Src kinase LCK to the vicinity of the TCR-CD3 complex. LCK then initiates different intracellular signaling pathways by phosphorylating various substrates ultimately leading to lymphokine production, motility, adhesion and activation of T-helper cells. In other cells such as macrophages or NK cells, plays a role in differentiation/activation, cytokine expression and cell migration in a TCR/LCK-independent pathway. Participates in the development of T-helper cells in the thymus and triggers the differentiation of monocytes into functional mature macrophages. Integral membrane glycoprotein that plays an essential role in the immune response and serves multiple functions in responses against both external and internal offenses.
Click to Show/Hide
|
|||||
| BioChemical Class |
Immunoglobulin
|
|||||
| UniProt ID | ||||||
| Sequence |
MNRGVPFRHLLLVLQLALLPAATQGKKVVLGKKGDTVELTCTASQKKSIQFHWKNSNQIK
ILGNQGSFLTKGPSKLNDRADSRRSLWDQGNFPLIIKNLKIEDSDTYICEVEDQKEEVQL LVFGLTANSDTHLLQGQSLTLTLESPPGSSPSVQCRSPRGKNIQGGKTLSVSQLELQDSG TWTCTVLQNQKKVEFKIDIVVLAFQKASSIVYKKEGEQVEFSFPLAFTVEKLTGSGELWW QAERASSSKSWITFDLKNKEVSVKRVTQDPKLQMGKKLPLHLTLPQALPQYAGSGNLTLA LEAKTGKLHQEVNLVVMRATQLQKNLTCEVWGPTSPKLMLSLKLENKEAKVSKREKAVWV LNPEAGMWQCLLSDSGQVLLESNIKVLPTWSTPVQPMALIVLGGVAGLLLFIGLGIFFCV RCRHRRRQAERMSQIKRLLSEKKTCQCPHRFQKTCSPI Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T53RK9 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Ibalizumab | Drug Info | Approved | Human immunodeficiency virus infection | [1] | |
| Clinical Trial Drug(s) | [+] 10 Clinical Trial Drugs | + | ||||
| 1 | Magrolimab | Drug Info | Phase 3 | Acute myeloid leukaemia | [2] | |
| 2 | TNX-355 | Drug Info | Phase 3 | Human immunodeficiency virus infection | [3] | |
| 3 | Zanolimumab | Drug Info | Phase 3 | Lymphoma | [4] | |
| 4 | BT-061 | Drug Info | Phase 2 | Asthma | [5], [6] | |
| 5 | Fluoropeptide vaccine | Drug Info | Phase 2 | Influenza virus infection | [7] | |
| 6 | CD4CAR | Drug Info | Phase 1 | T-cell leukaemia | [8] | |
| 7 | Combinectin | Drug Info | Phase 1 | Human immunodeficiency virus infection | [9] | |
| 8 | LIPO-4 | Drug Info | Phase 1 | Human immunodeficiency virus infection | [10] | |
| 9 | TMB-365 | Drug Info | Phase 1 | Human immunodeficiency virus-1 infection | [11] | |
| 10 | VRC07-523LS | Drug Info | Phase 1 | Human immunodeficiency virus-1 infection | [12] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | IDEC-151 | Drug Info | Discontinued in Phase 2 | Rheumatoid arthritis | [13] | |
| Mode of Action | [+] 3 Modes of Action | + | ||||
| Modulator | [+] 1 Modulator drugs | + | ||||
| 1 | Ibalizumab | Drug Info | [1] | |||
| Inhibitor | [+] 2 Inhibitor drugs | + | ||||
| 1 | Magrolimab | Drug Info | [14] | |||
| 2 | Combinectin | Drug Info | [20] | |||
| Binder | [+] 1 Binder drugs | + | ||||
| 1 | TNX-355 | Drug Info | [15] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
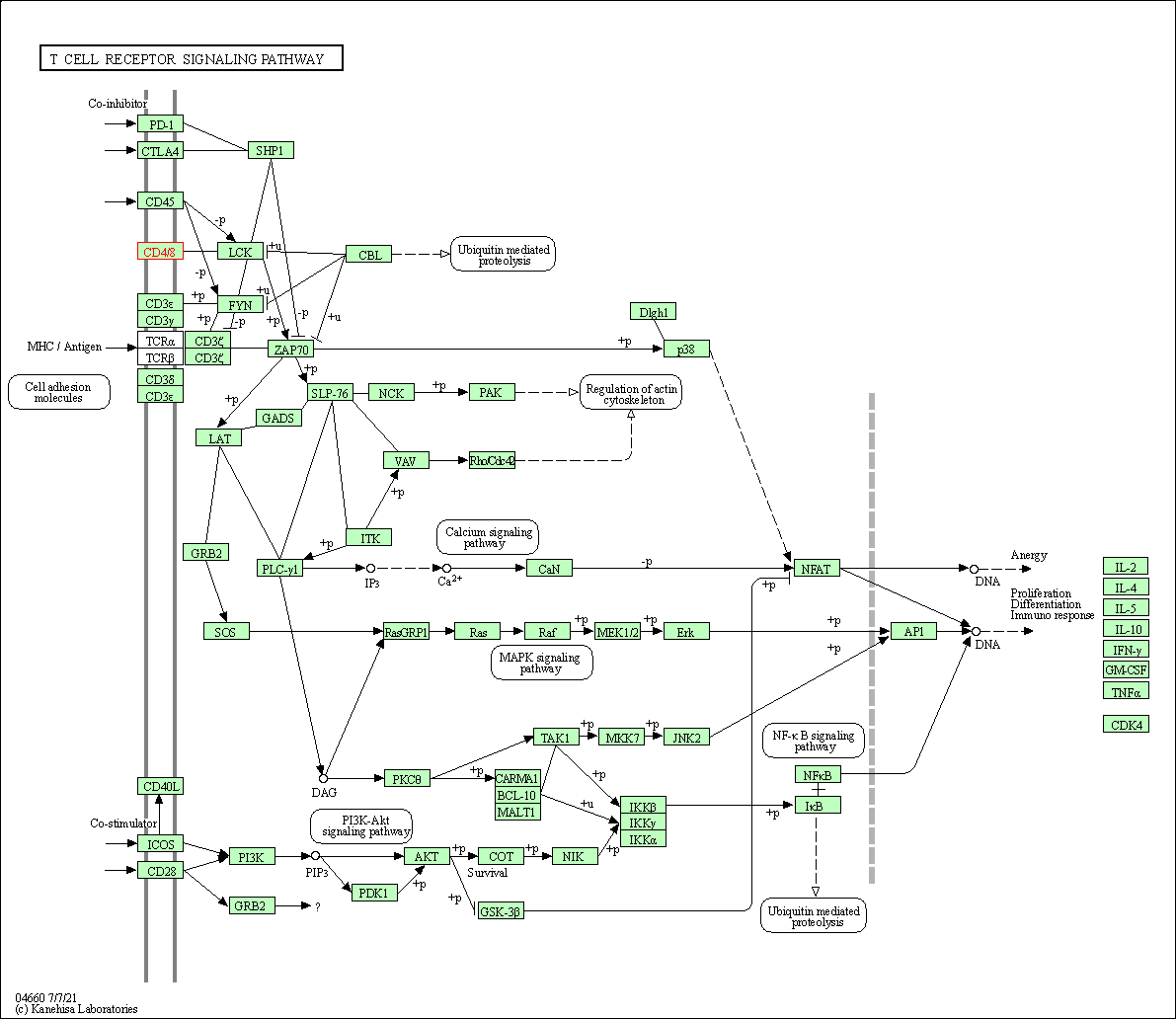
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
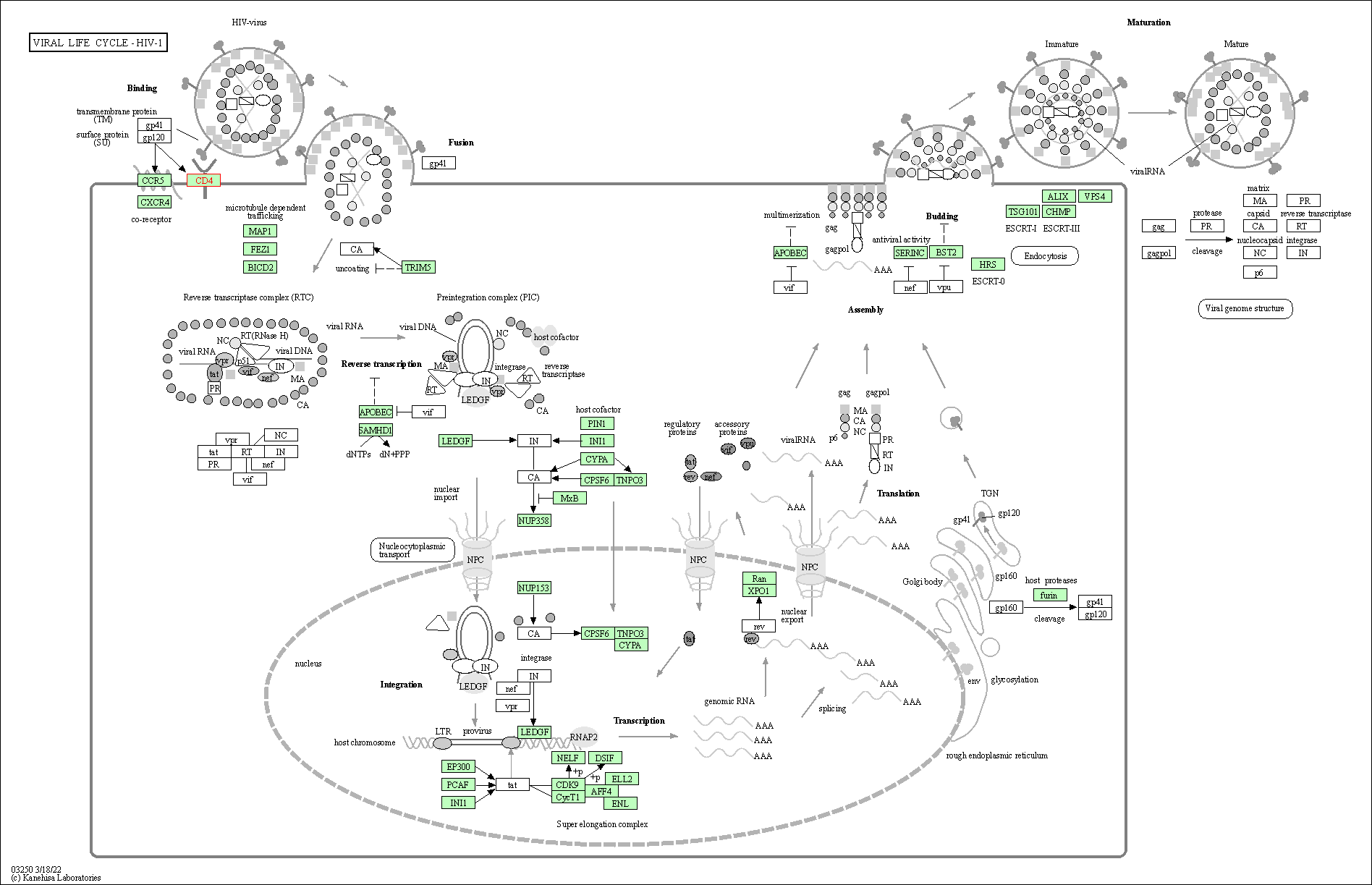
| Viral life cycle - HIV-1 | hsa03250 | Affiliated Target |

|
| Class: Genetic Information Processing => Information processing in viruses | Pathway Hierarchy | ||
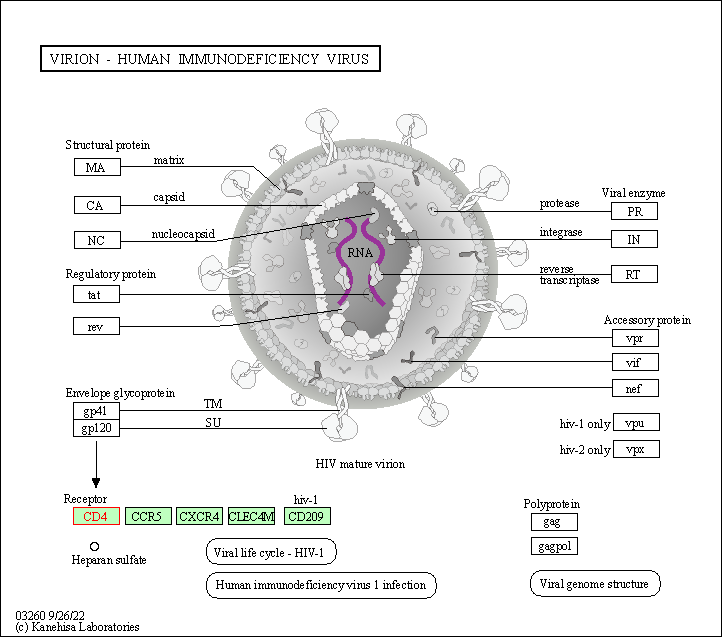
| Virion - Human immunodeficiency virus | hsa03260 | Affiliated Target |

|
| Class: Genetic Information Processing => Information processing in viruses | Pathway Hierarchy | ||
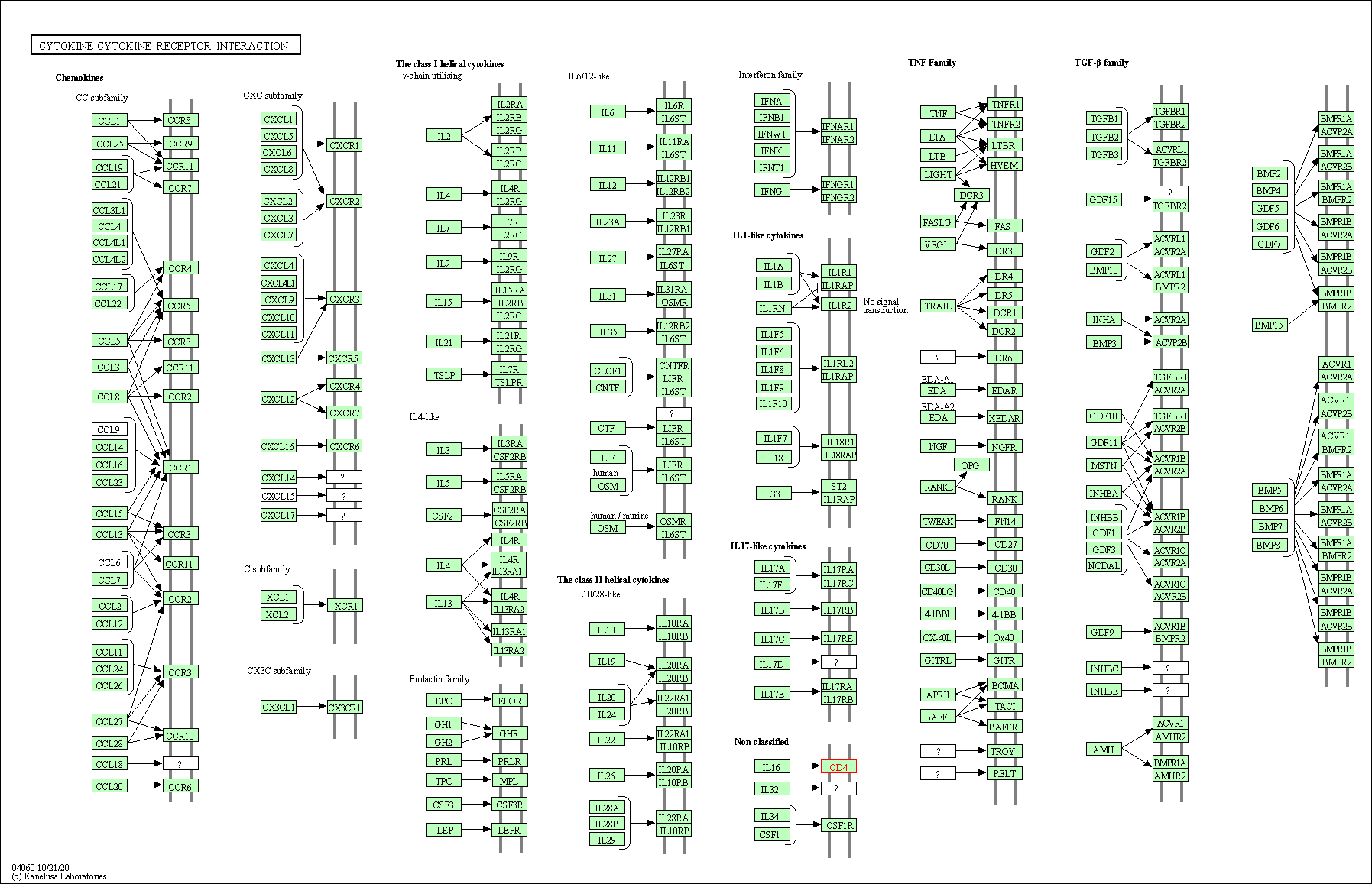
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
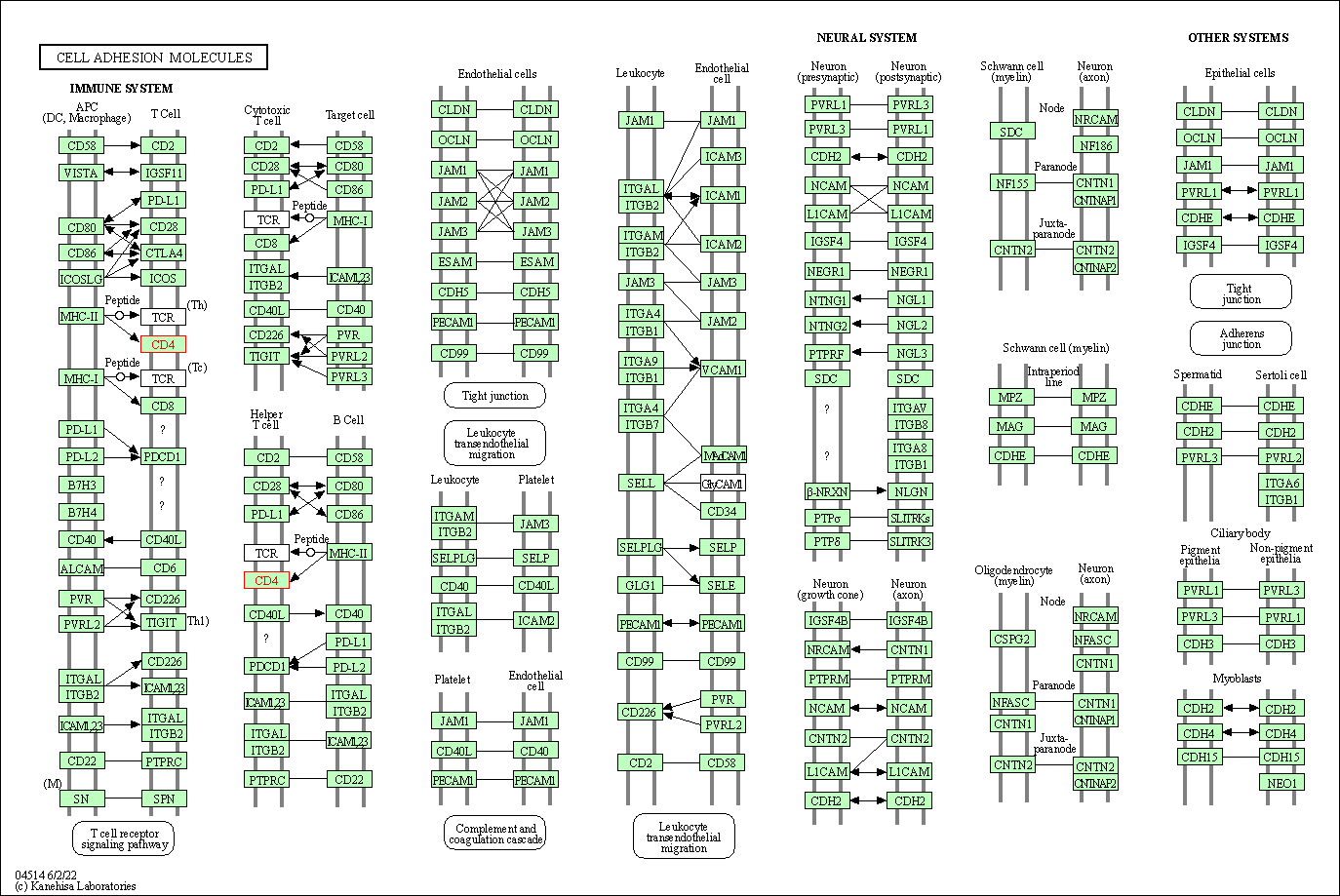
| Cell adhesion molecules | hsa04514 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
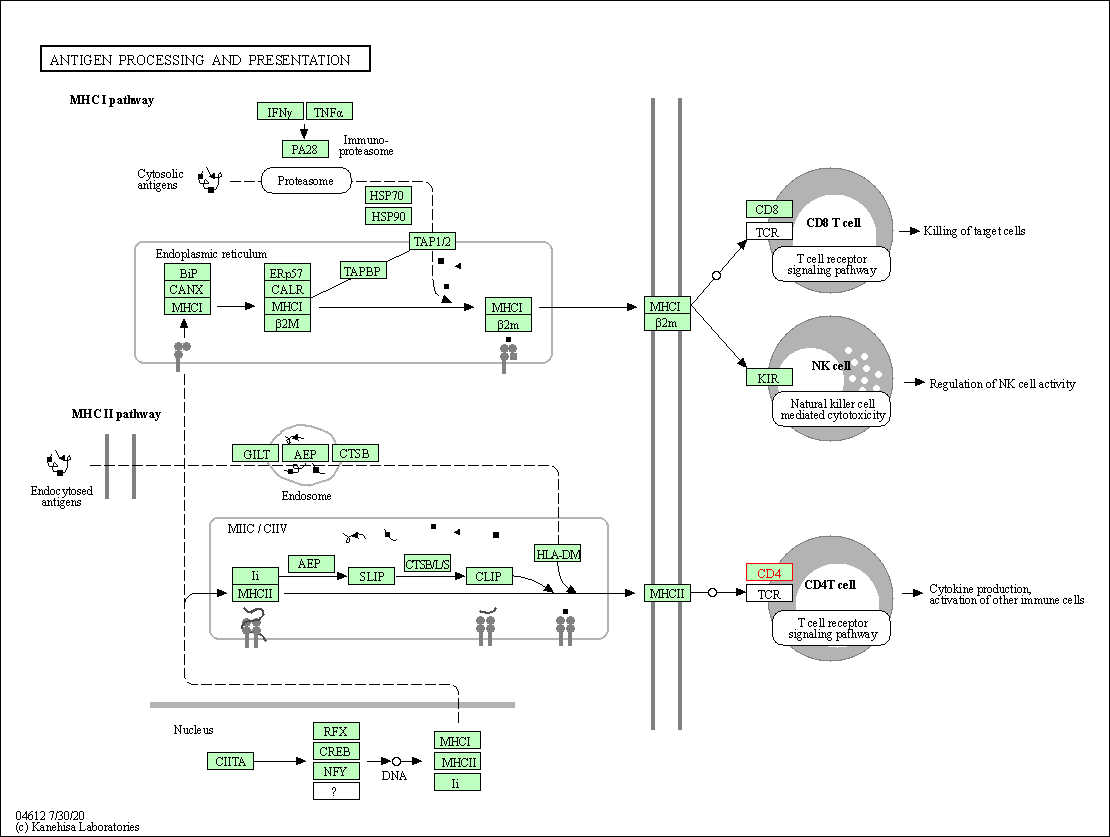
| Antigen processing and presentation | hsa04612 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
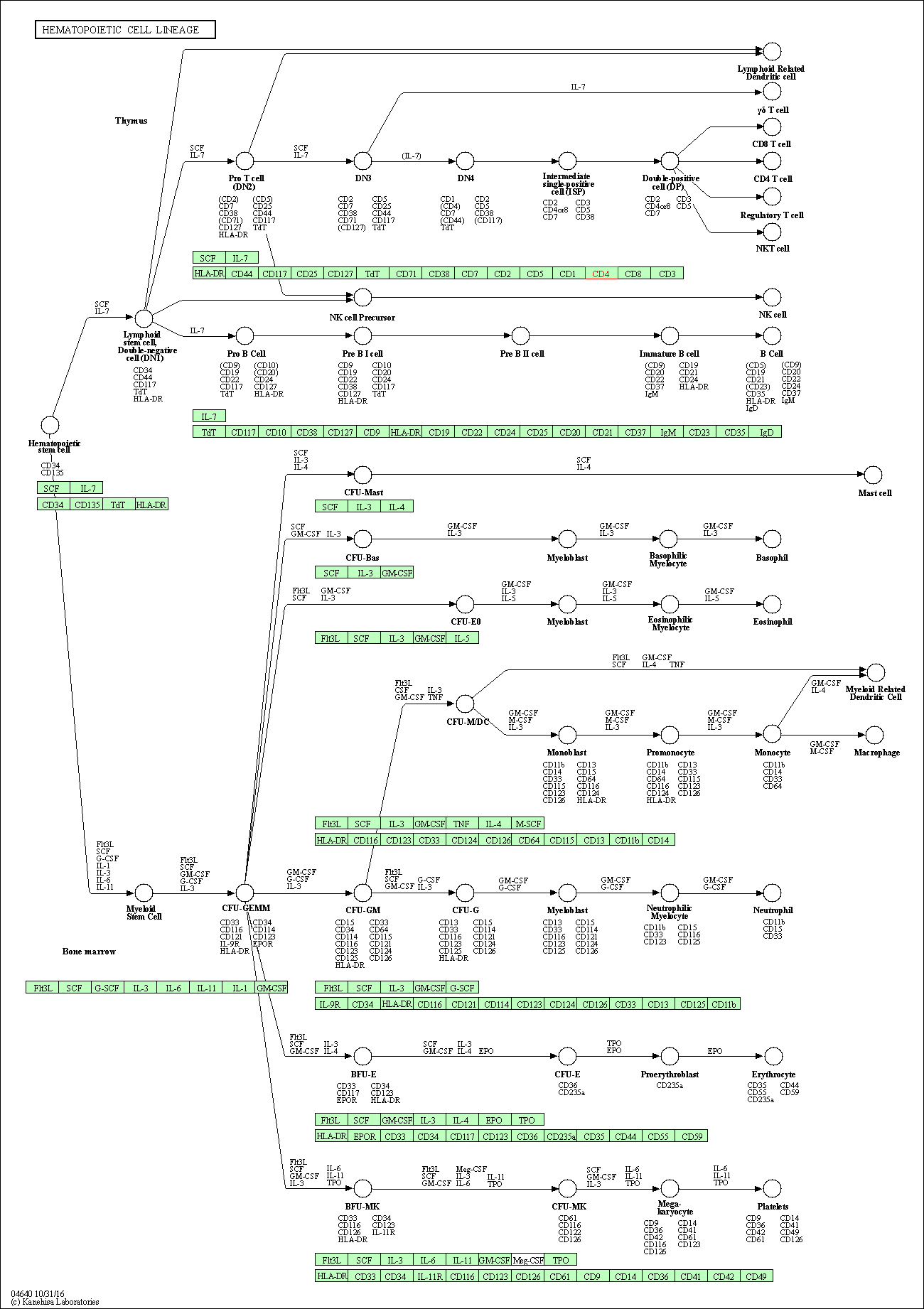
| Hematopoietic cell lineage | hsa04640 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
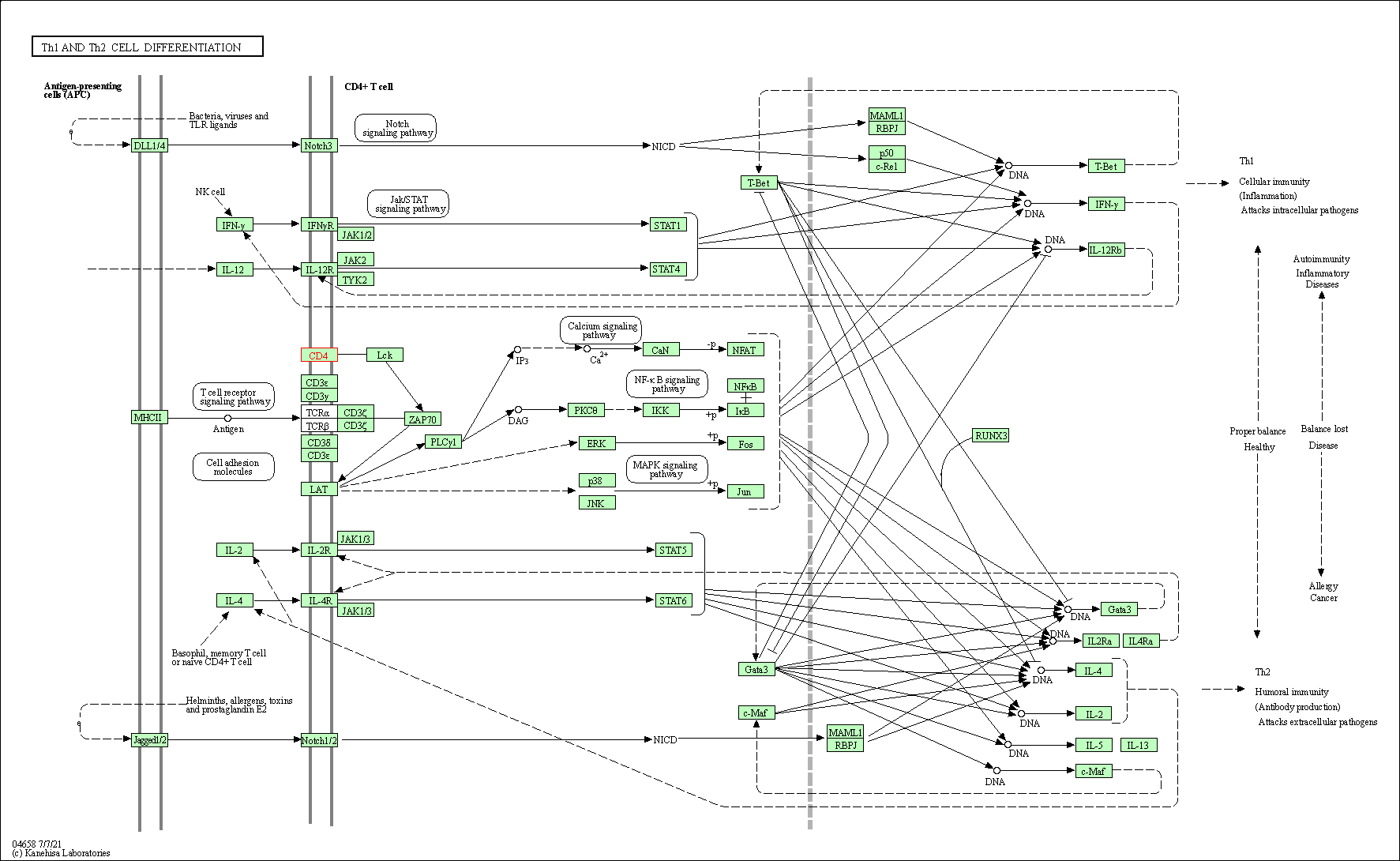
| Th1 and Th2 cell differentiation | hsa04658 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
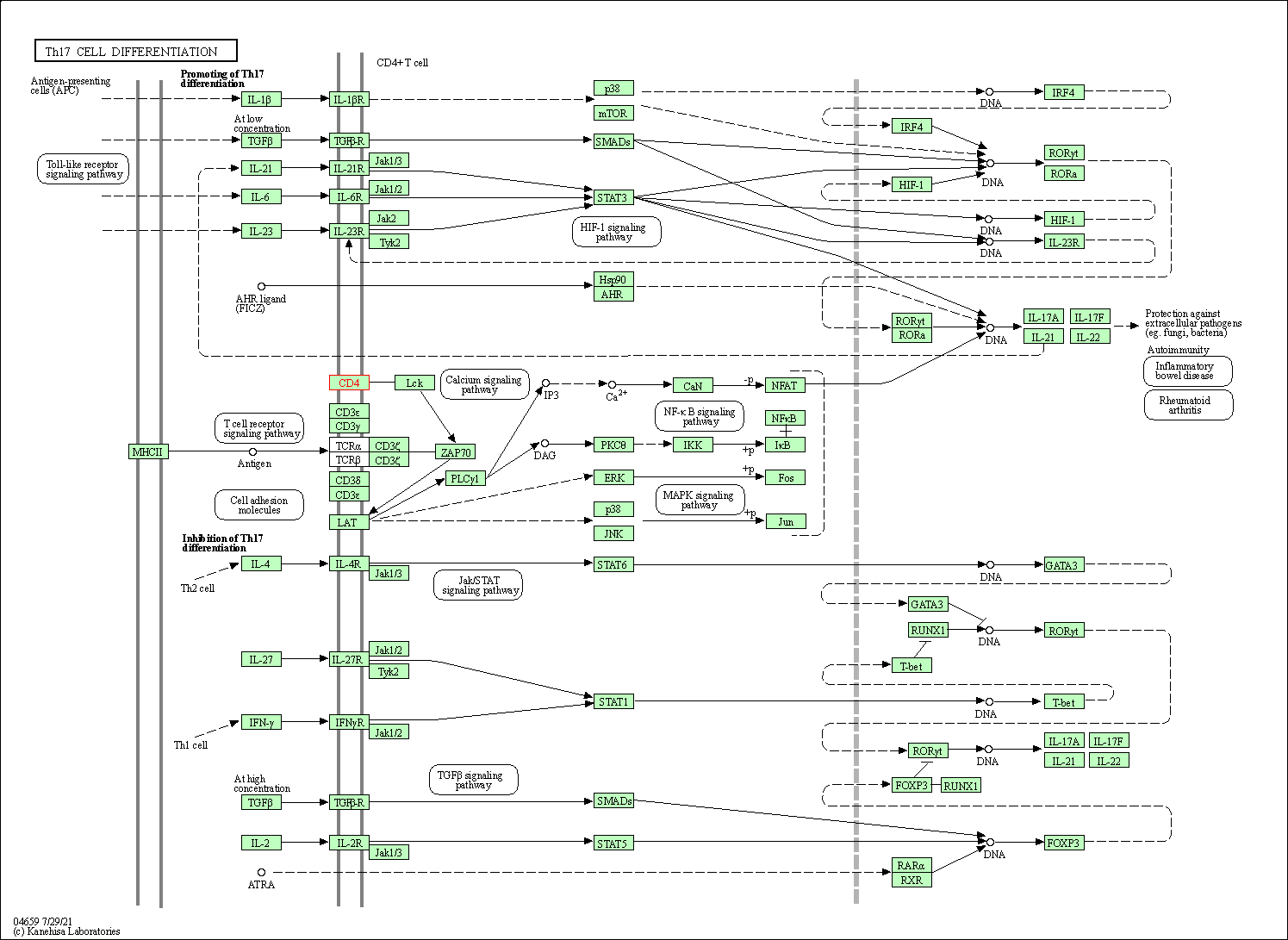
| Th17 cell differentiation | hsa04659 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| T cell receptor signaling pathway | hsa04660 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 43 | Degree centrality | 4.62E-03 | Betweenness centrality | 2.90E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.35E-01 | Radiality | 1.41E+01 | Clustering coefficient | 1.74E-01 |
| Neighborhood connectivity | 2.34E+01 | Topological coefficient | 5.54E-02 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating Transcription Factors | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | 2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89. | |||||
| REF 2 | ClinicalTrials.gov (NCT04778397) Study to Evaluate the Safety and Efficacy of Magrolimab in Combination With Azacitidine Versus Physician's Choice of Venetoclax in Combination With Azacitidine or Intensive Chemotherapy in Previously Untreated Adults With TP53 Mutant Acute Myeloid Leukemia (ENHANCE-2). U.S. National Institutes of Health. | |||||
| REF 3 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003716) | |||||
| REF 4 | ClinicalTrials.gov (NCT00127881) Study of Human Monoclonal Antibody to Treat Mycosis Fungoides and Sezary Syndrome. U.S. National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT01072383) Safety and Efficacy of Multiple Doses of BT061 in Patients With Moderate to Severe Chronic Plaque Psoriasis. U.S. National Institutes of Health. | |||||
| REF 6 | A specific CD4 epitope bound by tregalizumab mediates activation of regulatory T cells by a unique signaling pathway. Immunol Cell Biol. 2015 Apr;93(4):396-405. | |||||
| REF 7 | ClinicalTrials.gov (NCT01703923) An Exploratory Study of FP01 Lozenges in Subjects With Chronic Refractory Cough. U.S. National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT03829540) CD4CAR for CD4+ Leukemia and Lymphoma. U.S. National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT03984812) Evaluation of the Safety, Tolerability and Pharmacokinetics (PK) of GSK3732394 First-Time-in-Human (FTIH) Study. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT00121121) Safety of Intradermal Versus Intramuscular Administration of HIV Lipopeptides in HIV Uninfected Adult Volunteers. U.S. National Institutes of Health. | |||||
| REF 11 | ClinicalTrials.gov (NCT04027387) Dose Escalation Safety Study of TMB-365 in HIV-1 Infected Participants. U.S. National Institutes of Health. | |||||
| REF 12 | ClinicalTrials.gov (NCT03735849) Evaluating the Safety and Pharmacokinetics of VRC-HIVMAB075-00-AB (VRC07-523LS) in the Sera and Mucosae of Healthy, HIV-Uninfected Adult Participants. U.S. National Institutes of Health. | |||||
| REF 13 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007359) | |||||
| REF 14 | Current and emerging strategies for management of myelodysplastic syndromes. Blood Rev. 2021 Jul;48:100791. | |||||
| REF 15 | Progress in targeting HIV-1 entry. Drug Discov Today. 2005 Aug 15;10(16):1085-94. | |||||
| REF 16 | Clinical efficacy of zanolimumab (HuMax-CD4): two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood. 2007 Jun 1;109(11):4655-62. | |||||
| REF 17 | Tregalizumab (BT-061) increases regulatory T cell function. Boosting regulatory T-cell function with the humanized CD4-specific humanized monoclonal antibody Tregalizumab (BT-061). Immunol Cell Biol.2015 Apr;93(4):321-2. | |||||
| REF 18 | A novel peptide-based pan-influenza A vaccine: a double blind, randomised clinical trial of immunogenicity and safety. Vaccine. 2015 Jan 3;33(2):396-402. | |||||
| REF 19 | Clinical pipeline report, company report or official report of iCell Gene Therapeutics. | |||||
| REF 20 | Clinical pipeline report, company report or official report of ViiV Healthcare. | |||||
| REF 21 | Long-term CD4(+) and CD8(+) T-cell responses induced in HIV-uninfected volunteers following intradermal or intramuscular administration of an HIV-lipopeptide vaccine (ANRS VAC16). Vaccine. 2013 Sep 13;31(40):4406-15. | |||||
| REF 22 | Clinical pipeline report, company report or official report of TaiMed Biologics. | |||||
| REF 23 | Clinical pipeline report, company report or official report of TaiMed Biologics. | |||||
| REF 24 | Comparative pharmacodynamics of keliximab and clenoliximab in transgenic mice bearing human CD4. J Pharmacol Exp Ther. 2000 Apr;293(1):33-41. | |||||
| REF 25 | A small peptide (CEL-1000) derived from the beta-chain of the human major histocompatibility complex class II molecule induces complete protection against malaria in an antigen-independent manner. Antimicrob Agents Chemother. 2004 Jul;48(7):2455-63. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

