Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T13057
(Former ID: TTDR01263)
|
|||||
| Target Name |
Protein-tyrosine phosphatase SHP-2 (PTPN11)
|
|||||
| Synonyms |
Tyrosine-protein phosphatase non-receptor type 11; SHPTP2; SHP2; SHP-2; SH-PTP3; SH-PTP2; Protein-tyrosine phosphatase SHP2; Protein-tyrosine phosphatase 2C; Protein-tyrosine phosphatase 1D; PTP2C; PTP-2C; PTP-1D
Click to Show/Hide
|
|||||
| Gene Name |
PTPN11
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| Function |
Positively regulates MAPK signal transduction pathway. Dephosphorylates GAB1, ARHGAP35 and EGFR. Dephosphorylates ROCK2 at 'Tyr-722' resulting in stimulatation of its RhoA binding activity. Dephosphorylates CDC73. Acts downstream of various receptor and cytoplasmic protein tyrosine kinases to participate in the signal transduction from the cell surface to the nucleus.
Click to Show/Hide
|
|||||
| BioChemical Class |
Phosphoric monoester hydrolase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.1.3.48
|
|||||
| Sequence |
MTSRRWFHPNITGVEAENLLLTRGVDGSFLARPSKSNPGDFTLSVRRNGAVTHIKIQNTG
DYYDLYGGEKFATLAELVQYYMEHHGQLKEKNGDVIELKYPLNCADPTSERWFHGHLSGK EAEKLLTEKGKHGSFLVRESQSHPGDFVLSVRTGDDKGESNDGKSKVTHVMIRCQELKYD VGGGERFDSLTDLVEHYKKNPMVETLGTVLQLKQPLNTTRINAAEIESRVRELSKLAETT DKVKQGFWEEFETLQQQECKLLYSRKEGQRQENKNKNRYKNILPFDHTRVVLHDGDPNEP VSDYINANIIMPEFETKCNNSKPKKSYIATQGCLQNTVNDFWRMVFQENSRVIVMTTKEV ERGKSKCVKYWPDEYALKEYGVMRVRNVKESAAHDYTLRELKLSKVGQGNTERTVWQYHF RTWPDHGVPSDPGGVLDFLEEVHHKQESIMDAGPVVVHCSAGIGRTGTFIVIDILIDIIR EKGVDCDIDVPKTIQMVRSQRSGMVQTEAQYRFIYMAVQHYIETLQRRIEEEQKSKRKGH EYTNIKYSLADQTSGDQSPLPPCTPTPPCAEMREDSARVYENVGLMQQQKSFR Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T51K6D | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 9 Clinical Trial Drugs | + | ||||
| 1 | JAB-3312 | Drug Info | Phase 1/2 | Solid tumour/cancer | [2] | |
| 2 | A380 | Drug Info | Phase 1 | Aggressive cancer | [3] | |
| 3 | BBP-398 | Drug Info | Phase 1 | Solid tumour/cancer | [4] | |
| 4 | JAB-3068 | Drug Info | Phase 1 | Solid tumour/cancer | [5] | |
| 5 | PF-07284892 | Drug Info | Phase 1 | Aggressive cancer | [6] | |
| 6 | RG6433 | Drug Info | Phase 1 | Aggressive cancer | [7] | |
| 7 | RLY-1971 | Drug Info | Phase 1 | Solid tumour/cancer | [8] | |
| 8 | SAR442720 | Drug Info | Phase 1 | Solid tumour/cancer | [9] | |
| 9 | TNO155 | Drug Info | Phase 1 | Solid tumour/cancer | [1] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Inhibitor | [+] 11 Inhibitor drugs | + | ||||
| 1 | JAB-3312 | Drug Info | [10] | |||
| 2 | A380 | Drug Info | [3] | |||
| 3 | BBP-398 | Drug Info | [4] | |||
| 4 | JAB-3068 | Drug Info | [10] | |||
| 5 | PF-07284892 | Drug Info | [11] | |||
| 6 | RG6433 | Drug Info | [7] | |||
| 7 | RLY-1971 | Drug Info | [12] | |||
| 8 | SAR442720 | Drug Info | [13] | |||
| 9 | TNO155 | Drug Info | [1] | |||
| 10 | NSC-87877 | Drug Info | [14] | |||
| 11 | Dodecane-Trimethylamine | Drug Info | [15] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Dodecane-Trimethylamine | Ligand Info | |||||
| Structure Description | TYROSINE PHOSPHATASE SHP-2 | PDB:2SHP | ||||
| Method | X-ray diffraction | Resolution | 2.00 Å | Mutation | Yes | [16] |
| PDB Sequence |
KSRRWFHPNI
11 TGVEAENLLL21 TRGVDGSFLA31 RPSKSNPGDL41 TLSVRRNGAV51 THIKIQNTGD 61 YYDLYGGEKF71 ATLAELVQYY81 MEHHGQLKEK91 NGDVIELKYP101 LNCADPTSER 111 WFHGHLSGKE121 AEKLLTEKGK131 HGSFLVRESQ141 SHPGDFVLSV151 RTGDNDGKSK 166 VTHVMIRCQE176 LKYDVGGGER186 FDSLTDLVEH196 YKKNPMVETL206 GTVLQLKQPL 216 NTTRINAAEI226 ESRVRELSKG246 FWEEFETLQQ256 QECKLLYSRK266 EGQRQENKNK 276 NRYKNILPFD286 HTRVVLHDSD303 YINANIIMPK324 KSYIATQGCL334 QNTVNDFWRM 344 VFQENSRVIV354 MTTKEVERGK364 SKCVKYWPDE374 YALKEYGVMR384 VRNVKESAAH 394 DYTLRELKLS404 KVGQGNTERT414 VWQYHFRTWP424 DHGVPSDPGG434 VLDFLEEVHH 444 KQESIMDAGP454 VVVHCSAGIG464 RTGTFIVIDI474 LIDIIREKGV484 DCDIDVPKTI 494 QMVRSQRSGM504 VQTEAQYRSI514 YMAVQHYIET524 L
|
|||||
|
|
||||||
| Ligand Name: 4-(3-phenylphenyl)-N-(2,2,6,6-tetramethylpiperidin-4-yl)-1,3-thiazol-2-amine | Ligand Info | |||||
| Structure Description | The atomic structure of SHP2 E76A mutant in complex with allosteric inhibitor 9b | PDB:5XZR | ||||
| Method | X-ray diffraction | Resolution | 2.80 Å | Mutation | Yes | [17] |
| PDB Sequence |
MTSRRWFHPN
10 ITGVEAENLL20 LTRGVDGSFL30 ARPSKSNPGD40 FTLSVRRNGA50 VTHIKIQNTG 60 DYYDLYGGEK70 FATLAALVQY80 YMEHHGQLKE90 KNGDVIELKY100 PLNCADPTSE 110 RWFHGHLSGK120 EAEKLLTEKG130 KHGSFLVRES140 QSHPGDFVLS150 VRTGDDKGES 160 NDGKSKVTHV170 MIRCQELKYD180 VGGGERFDSL190 TDLVEHYKKN200 PMVETLGTVL 210 QLKQPLNTTR220 INAAEIESRV230 RELSKLAEKV243 KQGFWEEFET253 LQQQECKLLY 263 SRKEGQRQEN273 KNKNRYKNIL283 PFDHTRVVLH293 DGDPNEPVSD303 YINANIIMPK 325 SYIATQGCLQ335 NTVNDFWRMV345 FQENSRVIVM355 TTKEVERGKS365 KCVKYWPDEY 375 ALKEYGVMRV385 RNVKESAAHD395 YTLRELKLSK405 VGQGNTERTV415 WQYHFRTWPD 425 HGVPSDPGGV435 LDFLEEVHHK445 QESIMDAGPV455 VVHCSAGIGR465 TGTFIVIDIL 475 IDIIREKGVD485 CDIDVPKTIQ495 MVRSQRSGMV505 QTEAQYRFIY515 MAVQHYIETL 525 QRRIEEEQK
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
| Protein Name | Pfam ID | Percentage of Identity (%) | E value |
|---|---|---|---|
| Protein tyrosine phosphatase domain-containing protein 1 (PTPDC1) | 23.704 (32/135) | 1.00E-03 |
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Ras signaling pathway | hsa04014 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Phospholipase D signaling pathway | hsa04072 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Axon guidance | hsa04360 | Affiliated Target |

|
| Class: Organismal Systems => Development and regeneration | Pathway Hierarchy | ||
| C-type lectin receptor signaling pathway | hsa04625 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
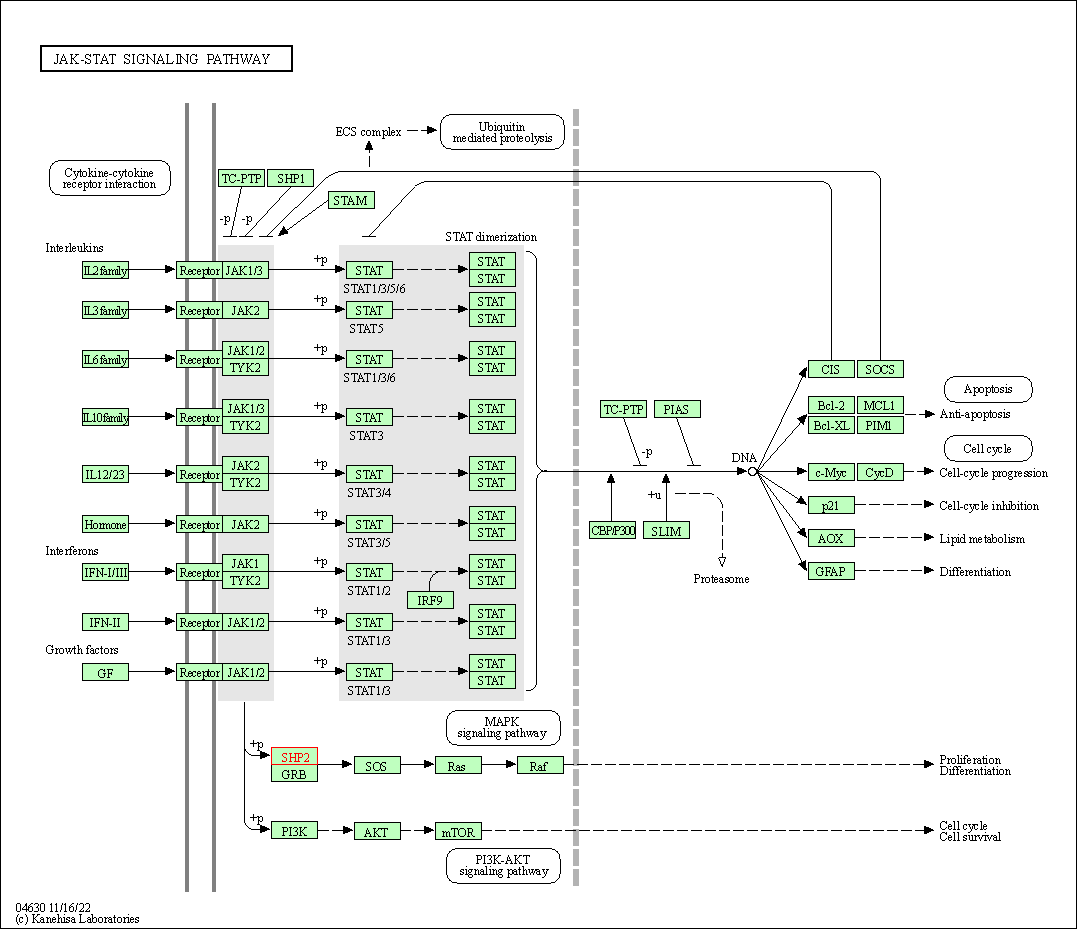
| JAK-STAT signaling pathway | hsa04630 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
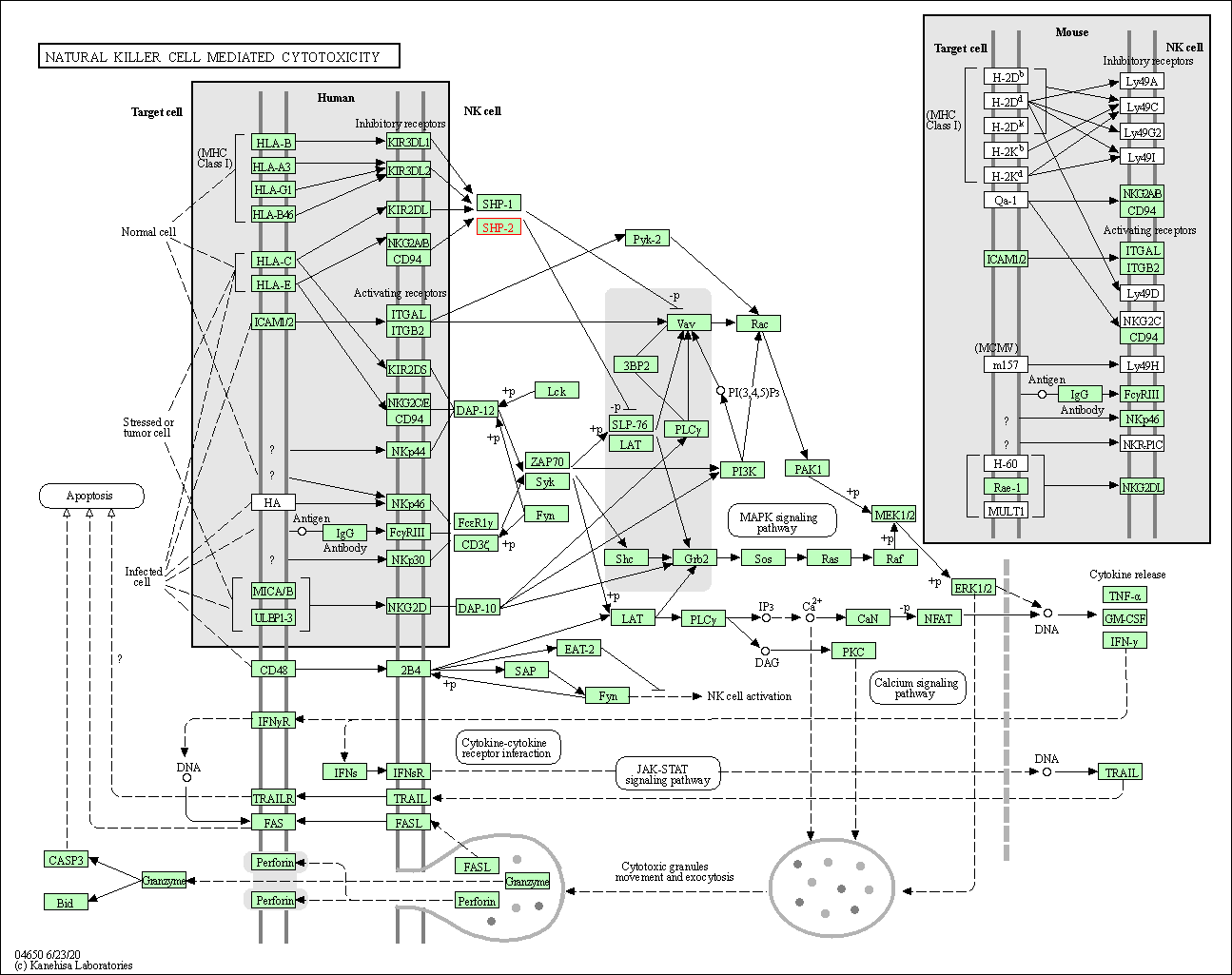
| Natural killer cell mediated cytotoxicity | hsa04650 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
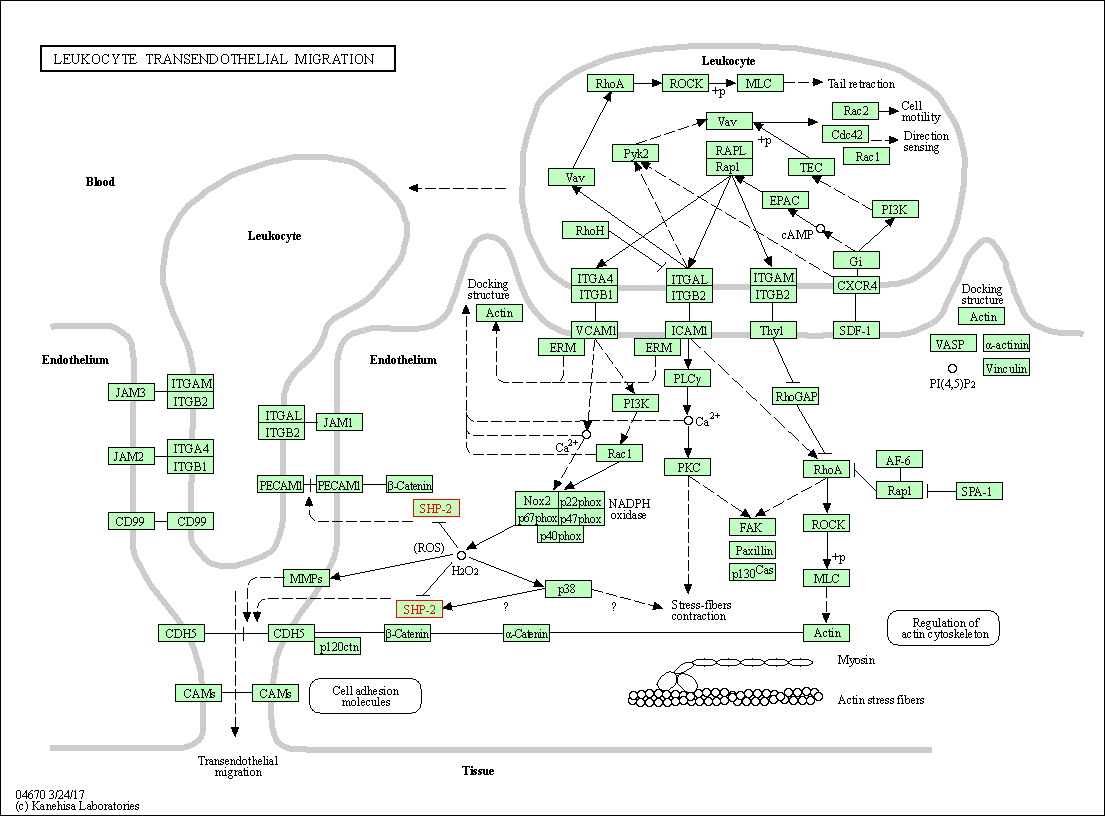
| Leukocyte transendothelial migration | hsa04670 | Affiliated Target |

|
| Class: Organismal Systems => Immune system | Pathway Hierarchy | ||
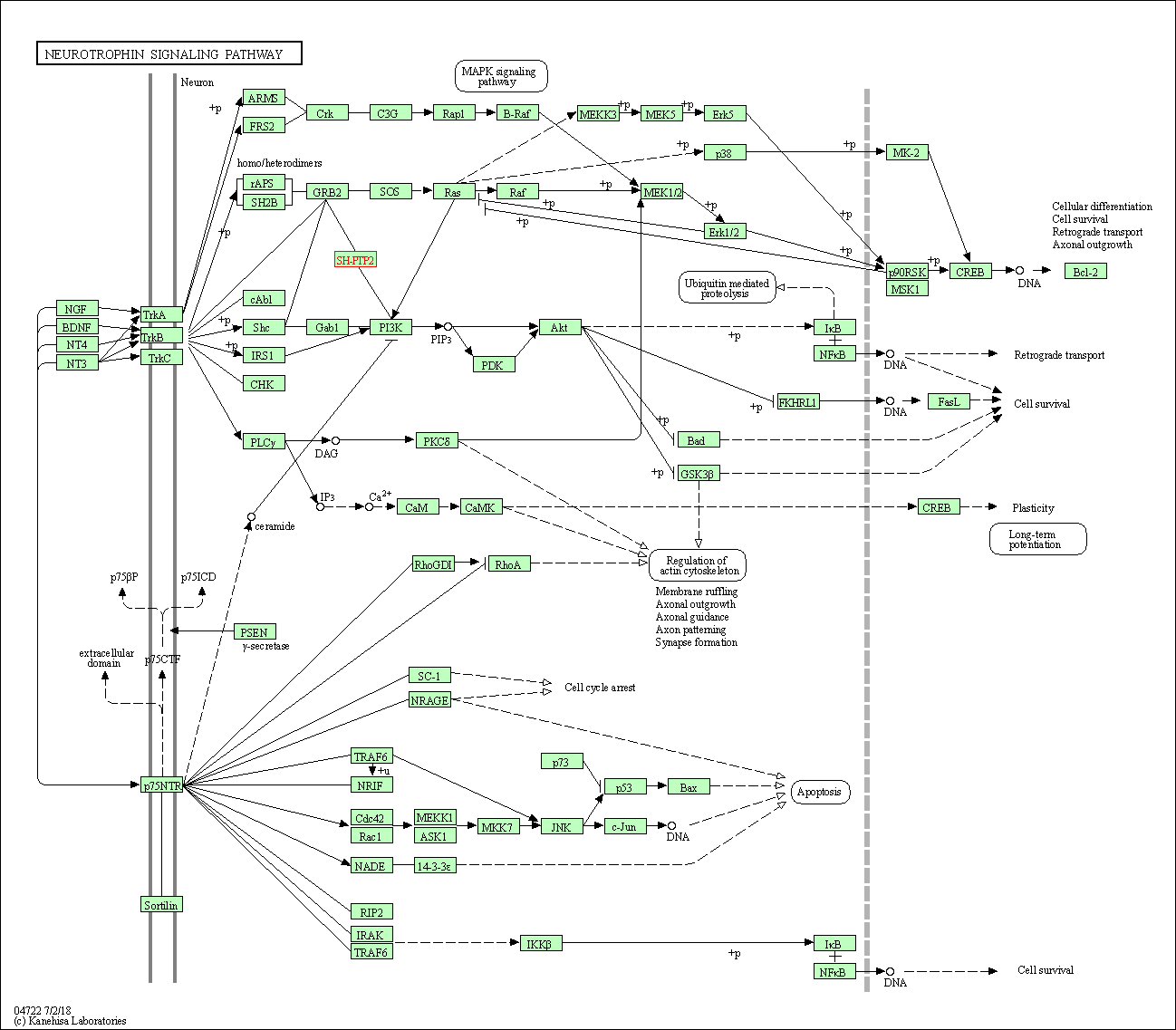
| Neurotrophin signaling pathway | hsa04722 | Affiliated Target |

|
| Class: Organismal Systems => Nervous system | Pathway Hierarchy | ||
| Adipocytokine signaling pathway | hsa04920 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Click to Show/Hide the Information of Affiliated Human Pathways | |||
| Degree | 85 | Degree centrality | 9.13E-03 | Betweenness centrality | 7.37E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.66E-01 | Radiality | 1.46E+01 | Clustering coefficient | 1.68E-01 |
| Neighborhood connectivity | 4.36E+01 | Topological coefficient | 3.64E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 2 | ClinicalTrials.gov (NCT04720976) JAB-3312 Activity in Adult Patients With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 3 | Clinical pipeline report, company report or official report of Klus Pharma | |||||
| REF 4 | ClinicalTrials.gov (NCT04528836) First-in-Human Study of the SHP2 Inhibitor BBP-398 in Patients With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 5 | ClinicalTrials.gov (NCT03518554) A First in Human, Dose Escalation Study of JAB-3068 (SHP2 Inhibitor) in Adult Patients With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT04800822) A PHASE 1, OPEN-LABEL, MULTI-CENTER, DOSE ESCALATION AND DOSE EXPANSION STUDY TO EVALUATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS, AND PRELIMINARY EVIDENCE OF ANTI-TUMOR ACTIVITY OF PF-07284892 (ARRY-558) AS A SINGLE AGENT AND IN COMBINATION THERAPY IN PARTICIPANTS WITH ADVANCED SOLID TUMORS. U.S.National Institutes of Health. | |||||
| REF 7 | Clinical pipeline report, company report or official report of Roche | |||||
| REF 8 | ClinicalTrials.gov (NCT04252339) RLY-1971 in Subjects With Advanced or Metastatic Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT04418661) Safety Study of SAR442720 in Combination With Pembrolizumab in Patients With Advanced Malignancies. U.S. National Institutes of Health. | |||||
| REF 10 | Clinical pipeline report, company report or official report of AbbVie. | |||||
| REF 11 | SHP2 Inhibition Sensitizes Diverse Oncogene-Addicted Solid Tumors to Re-treatment with Targeted Therapy. Cancer Discov. 2023 Aug 4;13(8):1789-1801. | |||||
| REF 12 | Clinical pipeline report, company report or official report of Relay Therapeutics. | |||||
| REF 13 | Clinical pipeline report, company report or official report of Revolution Medicines. | |||||
| REF 14 | Inhibitors of Src homology-2 domain containing protein tyrosine phosphatase-2 (Shp2) based on oxindole scaffolds. J Med Chem. 2008 Aug 28;51(16):4948-56. | |||||
| REF 15 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 16 | Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998 Feb 20;92(4):441-50. | |||||
| REF 17 | Allosteric Inhibitors of SHP2 with Therapeutic Potential for Cancer Treatment. J Med Chem. 2017 Dec 28;60(24):10205-10219. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

