Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T15344
(Former ID: TTDI02197)
|
|||||
| Target Name |
Activation-inducible TNFR family receptor (TNFRSF18)
|
|||||
| Synonyms |
UNQ319/PRO364; Tumor necrosis factor receptor superfamily member 18; Glucocorticoid-induced TNFR-related protein; GITR; CD357; AITR
Click to Show/Hide
|
|||||
| Gene Name |
TNFRSF18
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Melanoma [ICD-11: 2C30] | |||||
| 2 | Metastatic lymph node neoplasm [ICD-11: 2D60] | |||||
| 3 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| Function |
Seems to be involved in interactions between activated T-lymphocytes and endothelial cells and in the regulation of T-cell receptor-mediated cell death. Mediated NF-kappa-B activation via the TRAF2/NIK pathway. Receptor for TNFSF18.
Click to Show/Hide
|
|||||
| BioChemical Class |
Cytokine receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MAQHGAMGAFRALCGLALLCALSLGQRPTGGPGCGPGRLLLGTGTDARCCRVHTTRCCRD
YPGEECCSEWDCMCVQPEFHCGDPCCTTCRHHPCPPGQGVQSQGKFSFGFQCIDCASGTF SGGHEGHCKPWTDCTQFGFLTVFPGNKTHNAVCVPGSPPAEPLGWLTVVLLAVAACVLLL TSAQLGLHIWQLRSQCMWPRETQLLLEVPPSTEDARSCQFPEEERGERSAEEKGRLGDLW V Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 8 Clinical Trial Drugs | + | ||||
| 1 | INCAGN01876 | Drug Info | Phase 2 | Solid tumour/cancer | [2] | |
| 2 | INCAGN1876 | Drug Info | Phase 1/2 | Solid tumour/cancer | [3], [4] | |
| 3 | TRX-518 | Drug Info | Phase 1/2 | Solid tumour/cancer | [3], [4] | |
| 4 | AMG 228 | Drug Info | Phase 1 | Solid tumour/cancer | [5] | |
| 5 | ASP1951 | Drug Info | Phase 1 | Solid tumour/cancer | [6] | |
| 6 | MK-4166 | Drug Info | Phase 1 | Solid tumour/cancer | [7] | |
| 7 | REGN6569 | Drug Info | Phase 1 | Squamous head and neck cell carcinom | [8] | |
| 8 | TRX518 | Drug Info | Phase 1 | Solid tumour/cancer | [9] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Agonist | [+] 4 Agonist drugs | + | ||||
| 1 | INCAGN01876 | Drug Info | [10] | |||
| 2 | INCAGN1876 | Drug Info | [3] | |||
| 3 | AMG 228 | Drug Info | [11] | |||
| 4 | ASP1951 | Drug Info | [6] | |||
| Inhibitor | [+] 1 Inhibitor drugs | + | ||||
| 1 | REGN6569 | Drug Info | [13] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
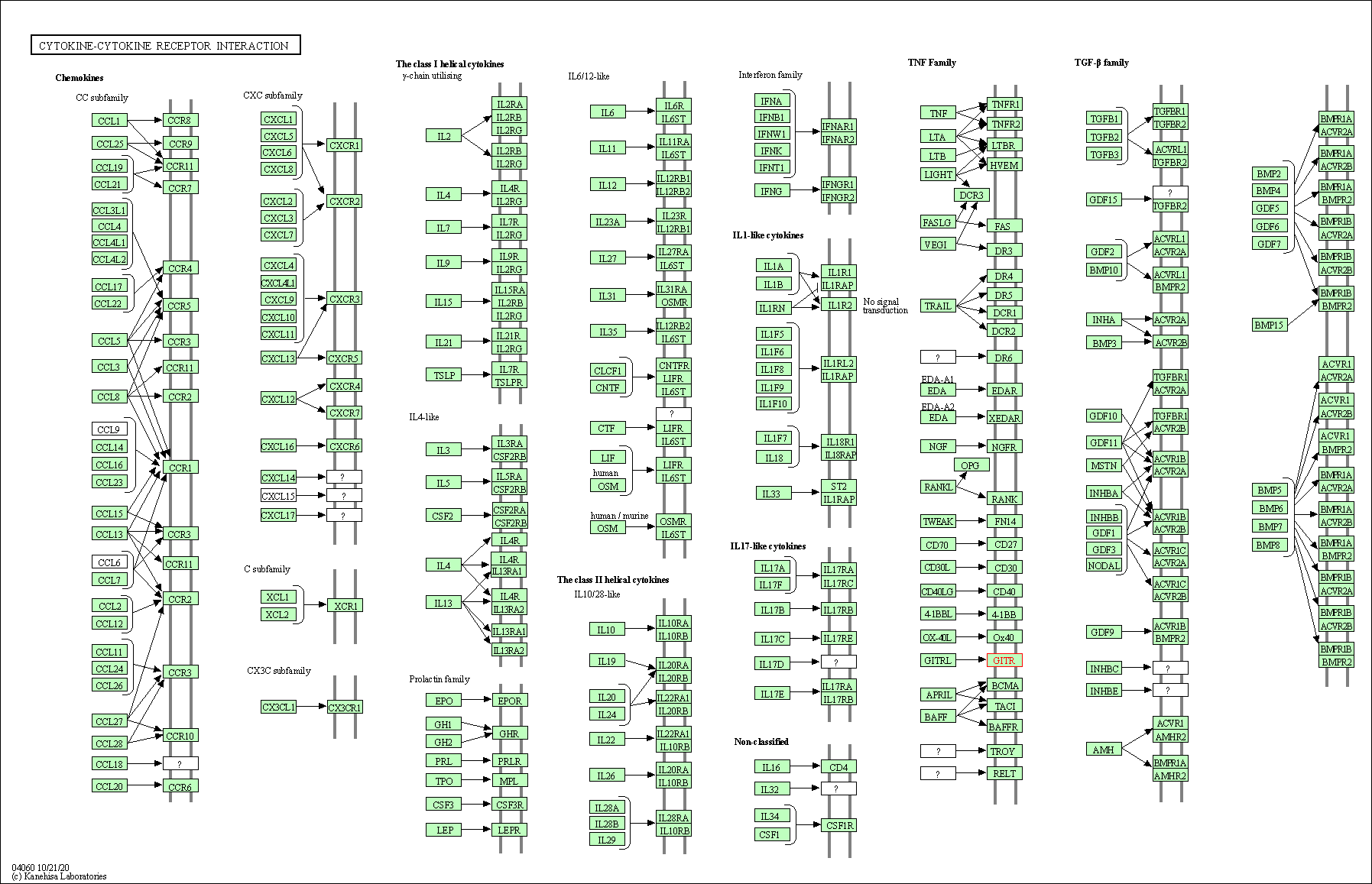
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Cytokine-cytokine receptor interaction | hsa04060 | Affiliated Target |

|
| Class: Environmental Information Processing => Signaling molecules and interaction | Pathway Hierarchy | ||
| Degree | 2 | Degree centrality | 2.15E-04 | Betweenness centrality | 2.02E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.92E-01 | Radiality | 1.33E+01 | Clustering coefficient | 0.00E+00 |
| Neighborhood connectivity | 1.00E+01 | Topological coefficient | 5.00E-01 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 1 KEGG Pathways | + | ||||
| 1 | Cytokine-cytokine receptor interaction | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | TCR Signaling Pathway | |||||
| PID Pathway | [+] 2 PID Pathways | + | ||||
| 1 | Downstream signaling in naï | |||||
| 2 | ||||||
| Reactome | [+] 1 Reactome Pathways | + | ||||
| 1 | TNFs bind their physiological receptors | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Immuno-oncology moves beyond PD-1. Nat Biotechnol. 2015 Jul;33(7):673-5. | |||||
| REF 2 | ClinicalTrials.gov (NCT03277352) INCAGN01876 in Combination With Immune Therapies in Subjects With Advanced or Metastatic Malignancies. U.S. National Institutes of Health. | |||||
| REF 3 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 4 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 5 | ClinicalTrials.gov (NCT02437916) Safety Study of AMG 228 to Treat Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT03799003) A Study of ASP1951 in Subjects With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT02132754) Study of MK-4166 in Participants With Advanced Solid Tumors (MK-4166-001). U.S. National Institutes of Health. | |||||
| REF 8 | ClinicalTrials.gov (NCT04465487) Study of REGN6569 and Cemiplimab in Adult Patients With Advanced Solid Tumor Malignancies. U.S. National Institutes of Health. | |||||
| REF 9 | ClinicalTrials.gov (NCT02628574) Phase 1 Open-label Study of TRX518 Monotherapy and TRX518 in Combination With Gemcitabine, Pembrolizumab, or Nivolumab. U.S. National Institutes of Health. | |||||
| REF 10 | Clinical pipeline report, company report or official report of Agenus. | |||||
| REF 11 | Dose escalation results from a first-in-human, phase 1 study of glucocorticoid-induced TNF receptor-related protein agonist AMG 228 in patients with advanced solid tumors. J Immunother Cancer. 2018 Sep 25;6(1):93. | |||||
| REF 12 | Immuno-oncology moves beyond PD-1. Nat Biotechnol. 2015 Jul;33(7):673-5. | |||||
| REF 13 | Clinical pipeline report, company report or official report of Regeneron Pharmaceuticals. | |||||
| REF 14 | Clinical pipeline report, company report or official report of Leap Therapeutics. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

