Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T23145
(Former ID: TTDS00419)
|
|||||
| Target Name |
Cholesterol desmolase (CYP11A1)
|
|||||
| Synonyms |
P450(scc); Cytochrome P450(scc); Cytochrome P450 11A1; Cholesterol side-chain cleavage enzyme, mitochondrial; CYPXIA1; CYP11A
Click to Show/Hide
|
|||||
| Gene Name |
CYP11A1
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Cushing syndrome [ICD-11: 5A70] | |||||
| Function |
Catalyzes the side-chain cleavage reaction of cholesterol to pregnenolone, the precursor of most steroid hormones.
Click to Show/Hide
|
|||||
| BioChemical Class |
Paired donor oxygen oxidoreductase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 1.14.15.6
|
|||||
| Sequence |
MLAKGLPPRSVLVKGCQTFLSAPREGLGRLRVPTGEGAGISTRSPRPFNEIPSPGDNGWL
NLYHFWRETGTHKVHLHHVQNFQKYGPIYREKLGNVESVYVIDPEDVALLFKSEGPNPER FLIPPWVAYHQYYQRPIGVLLKKSAAWKKDRVALNQEVMAPEATKNFLPLLDAVSRDFVS VLHRRIKKAGSGNYSGDISDDLFRFAFESITNVIFGERQGMLEEVVNPEAQRFIDAIYQM FHTSVPMLNLPPDLFRLFRTKTWKDHVAAWDVIFSKADIYTQNFYWELRQKGSVHHDYRG ILYRLLGDSKMSFEDIKANVTEMLAGGVDTTSMTLQWHLYEMARNLKVQDMLRAEVLAAR HQAQGDMATMLQLVPLLKASIKETLRLHPISVTLQRYLVNDLVLRDYMIPAKTLVQVAIY ALGREPTFFFDPENFDPTRWLSKDKNITYFRNLGFGWGVRQCLGRRIAELEMTIFLINML ENFRVEIQHLSDVGTTFNLILMPEKPISFTFWPFNQEATQQ Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| HIT2.0 ID | T85S9Z | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 1 Approved Drugs | + | ||||
| 1 | Aminoglutethimide | Drug Info | Approved | Cushing disease | [1], [2] | |
| Clinical Trial Drug(s) | [+] 1 Clinical Trial Drugs | + | ||||
| 1 | MK-5684 | Drug Info | Phase 1/2 | Prostate cancer | [3] | |
| Mode of Action | [+] 1 Modes of Action | + | ||||
| Inhibitor | [+] 4 Inhibitor drugs | + | ||||
| 1 | Aminoglutethimide | Drug Info | [1] | |||
| 2 | MK-5684 | Drug Info | [4] | |||
| 3 | 3-(4-Amino-phenyl)-3-butyl-piperidine-2,6-dione | Drug Info | [5] | |||
| 4 | 3-(4-Amino-phenyl)-3-pentyl-piperidine-2,6-dione | Drug Info | [5] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Cholesterol | Ligand Info | |||||
| Structure Description | Crystal structure of human CYP11A1 in complex with cholesterol | PDB:3N9Y | ||||
| Method | X-ray diffraction | Resolution | 2.10 Å | Mutation | No | [6] |
| PDB Sequence |
SPRPFNEIPS
14 PGDNGWLNLY24 HFWRETGTHK34 VHLHHVQNFQ44 KYGPIYREKL54 GNVESVYVID 64 PEDVALLFKS74 EGPNPERFLI84 PPWVAYHQYY94 QRPIGVLLKK104 SAAWKKDRVA 114 LNQEVMAPEA124 TKNFLPLLDA134 VSRDFVSVLH144 RRIKKAGSGN154 YSGDISDDLF 164 RFAFESITNV174 IFGERQGMLE184 EVVNPEAQRF194 IDAIYQMFHT204 SVPMLNLPPD 214 LFRLFRTKTW224 KDHVAAWDVI234 FSKADIYTQN244 FYWELRQKGS254 VHHDYRGILY 264 RLLGDSKMSF274 EDIKANVTEM284 LAGGVDTTSM294 TLQWHLYEMA304 RNLKVQDMLR 314 AEVLAARHQA324 QGDMATMLQL334 VPLLKASIKE344 TLRLHPISVT354 LQRYLVNDLV 364 LRDYMIPAKT374 LVQVAIYALG384 REPTFFFDPE394 NFDPTRWLSK404 DKNITYFRNL 414 GFGWGVRQCL424 GRRIAELEMT434 IFLINMLENF444 RVEIQHLSDV454 GTTFNLILMP 464 EKPISFTFWP474 F
|
|||||
|
|
ARG81
3.970
PHE82
3.566
ILE84
3.752
TRP87
4.070
LEU101
3.727
MET201
3.753
PHE202
3.857
TRP231
4.505
GLU283
3.714
ALA286
3.979
GLY287
3.834
|
|||||
| Ligand Name: 22R-hydroxycholesterol | Ligand Info | |||||
| Structure Description | Crystal structure of human CYP11A1 in complex with 22-hydroxycholesterol | PDB:3N9Z | ||||
| Method | X-ray diffraction | Resolution | 2.17 Å | Mutation | No | [6] |
| PDB Sequence |
PRPFNEIPSP
15 GDNGWLNLYH25 FWRETGTHKV35 HLHHVQNFQK45 YGPIYREKLG55 NVESVYVIDP 65 EDVALLFKSE75 GPNPERFLIP85 PWVAYHQYYQ95 RPIGVLLKKS105 AAWKKDRVAL 115 NQEVMAPEAT125 KNFLPLLDAV135 SRDFVSVLHR145 RIKKAGSGNY155 SGDISDDLFR 165 FAFESITNVI175 FGERQGMLEE185 VVNPEAQRFI195 DAIYQMFHTS205 VPMLNLPPDL 215 FRLFRTKTWK225 DHVAAWDVIF235 SKADIYTQNF245 YWELRQKGSV255 HHDYRGILYR 265 LLGDSKMSFE275 DIKANVTEML285 AGGVDTTSMT295 LQWHLYEMAR305 NLKVQDMLRA 315 EVLAARHQAQ325 GDMATMLQLV335 PLLKASIKET345 LRLHPISVTL355 QRYLVNDLVL 365 RDYMIPAKTL375 VQVAIYALGR385 EPTFFFDPEN395 FDPTRWLSKD405 KNITYFRNLG 415 FGWGVRQCLG425 RRIAELEMTI435 FLINMLENFR445 VEIQHLSDVG455 TTFNLILMPE 465 KPISFTFWPF475
|
|||||
|
|
ARG81
3.994
PHE82
3.573
ILE84
3.803
TRP87
4.394
LEU101
3.801
MET201
4.033
PHE202
3.584
GLU283
3.468
ALA286
4.155
GLY287
3.867
THR291
3.836
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
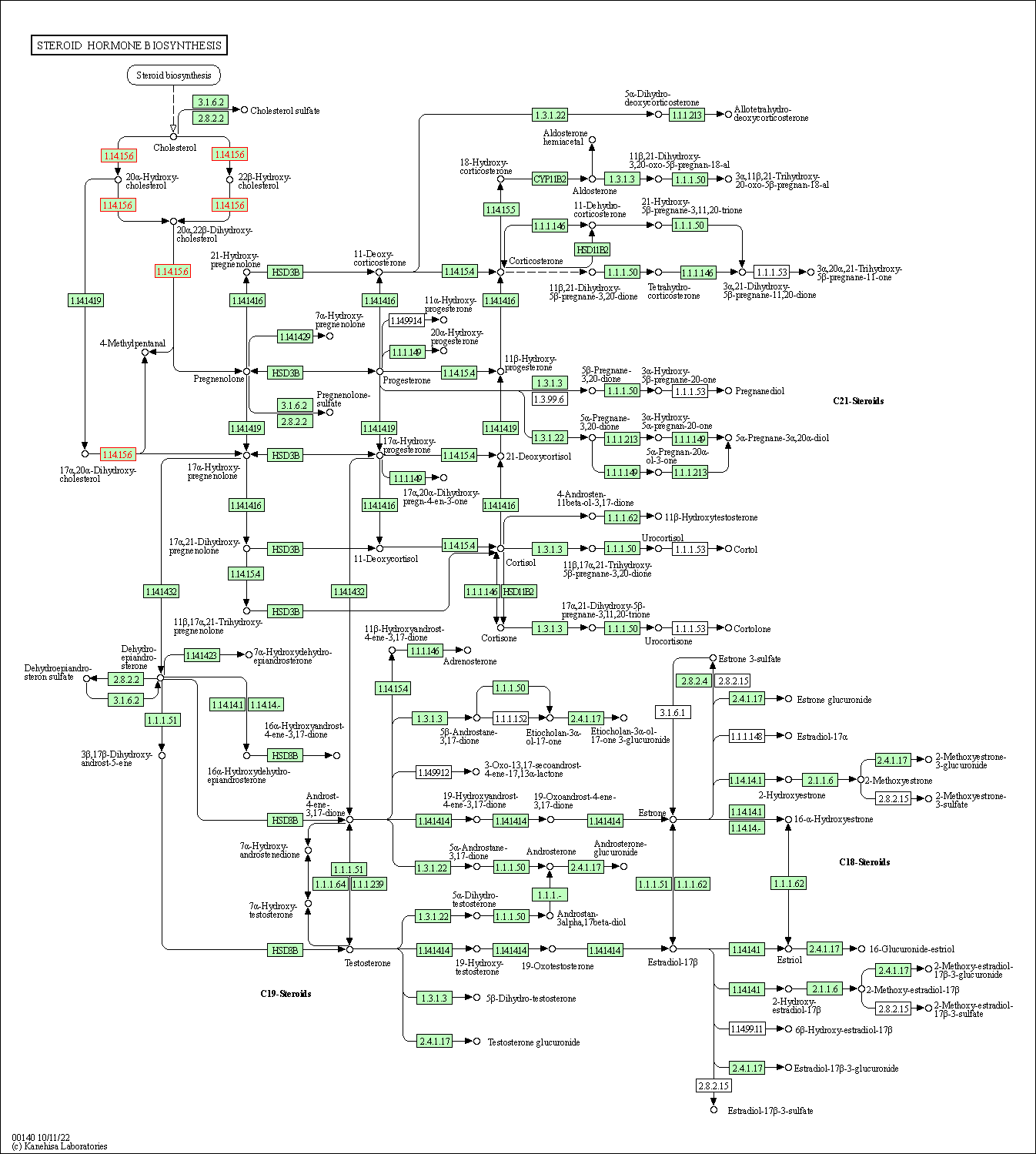
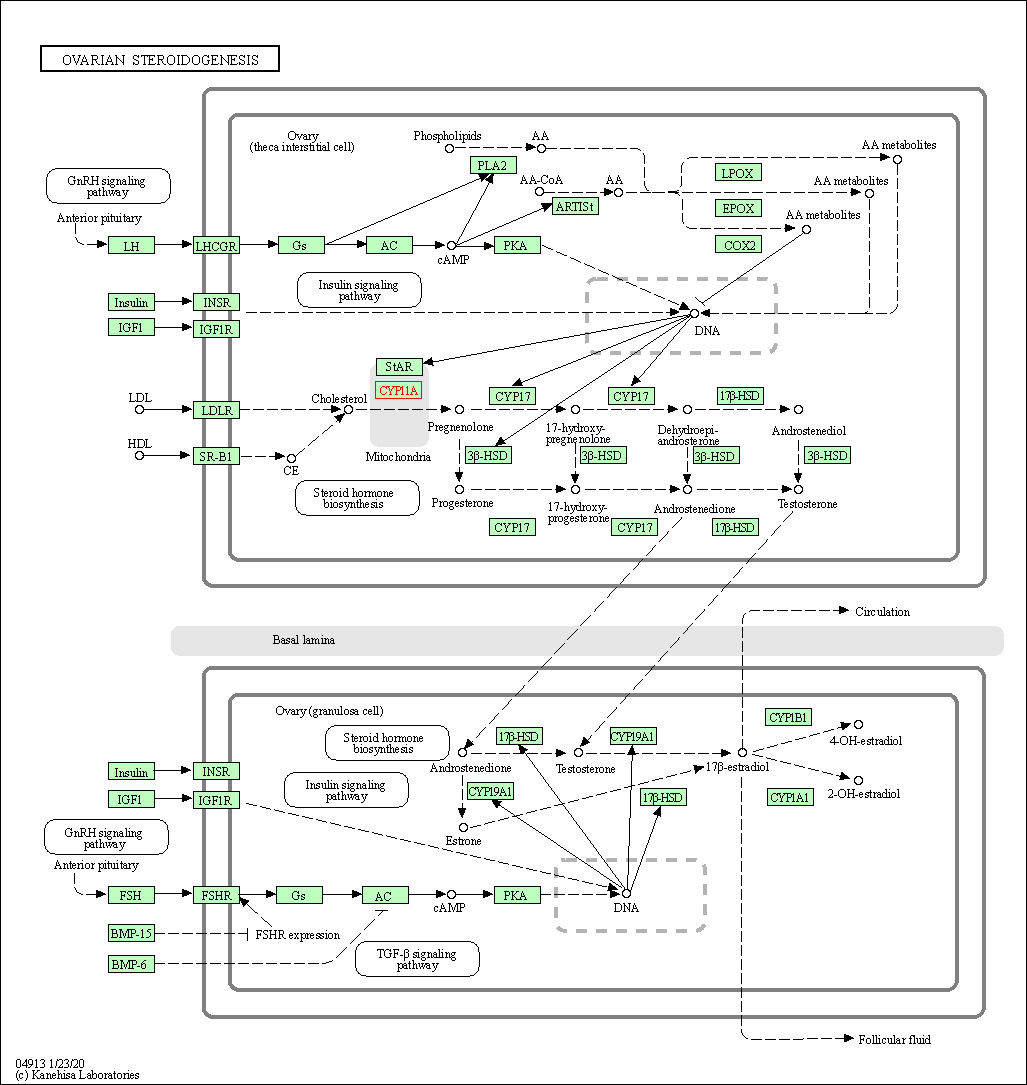
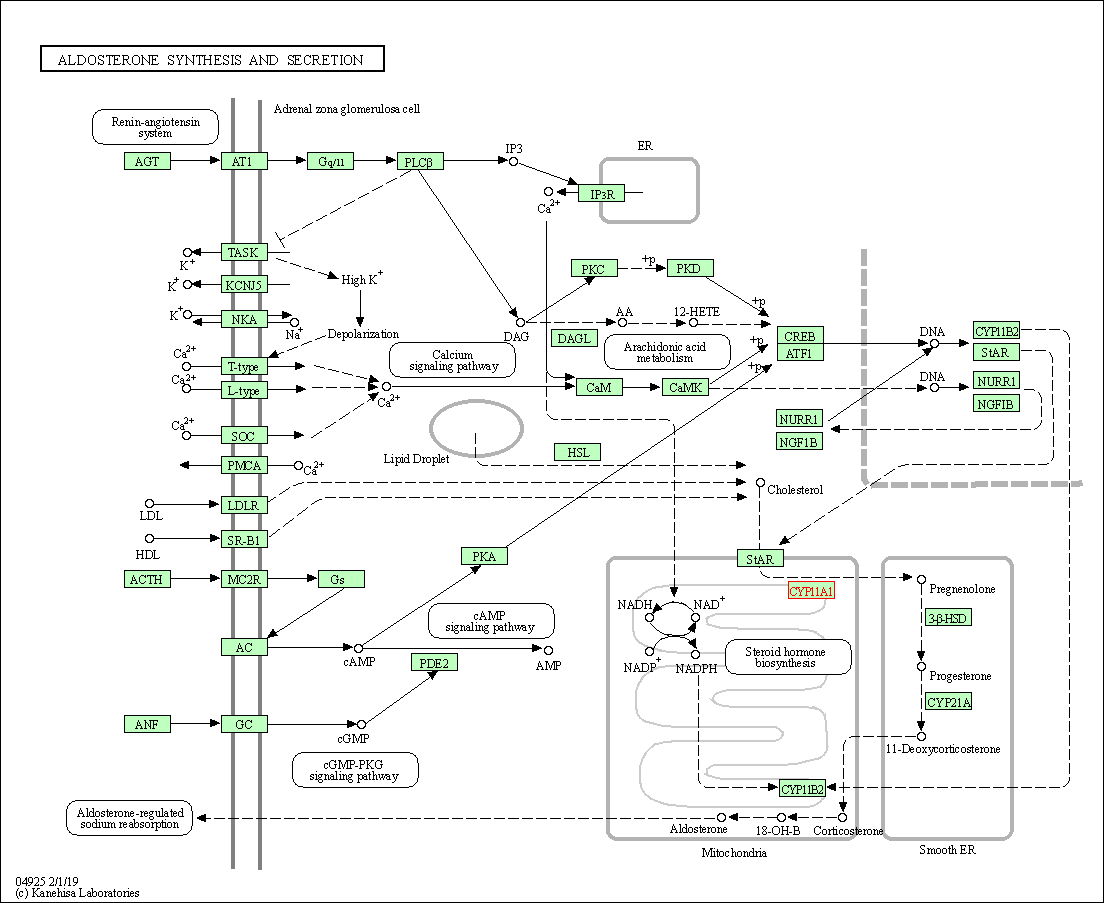
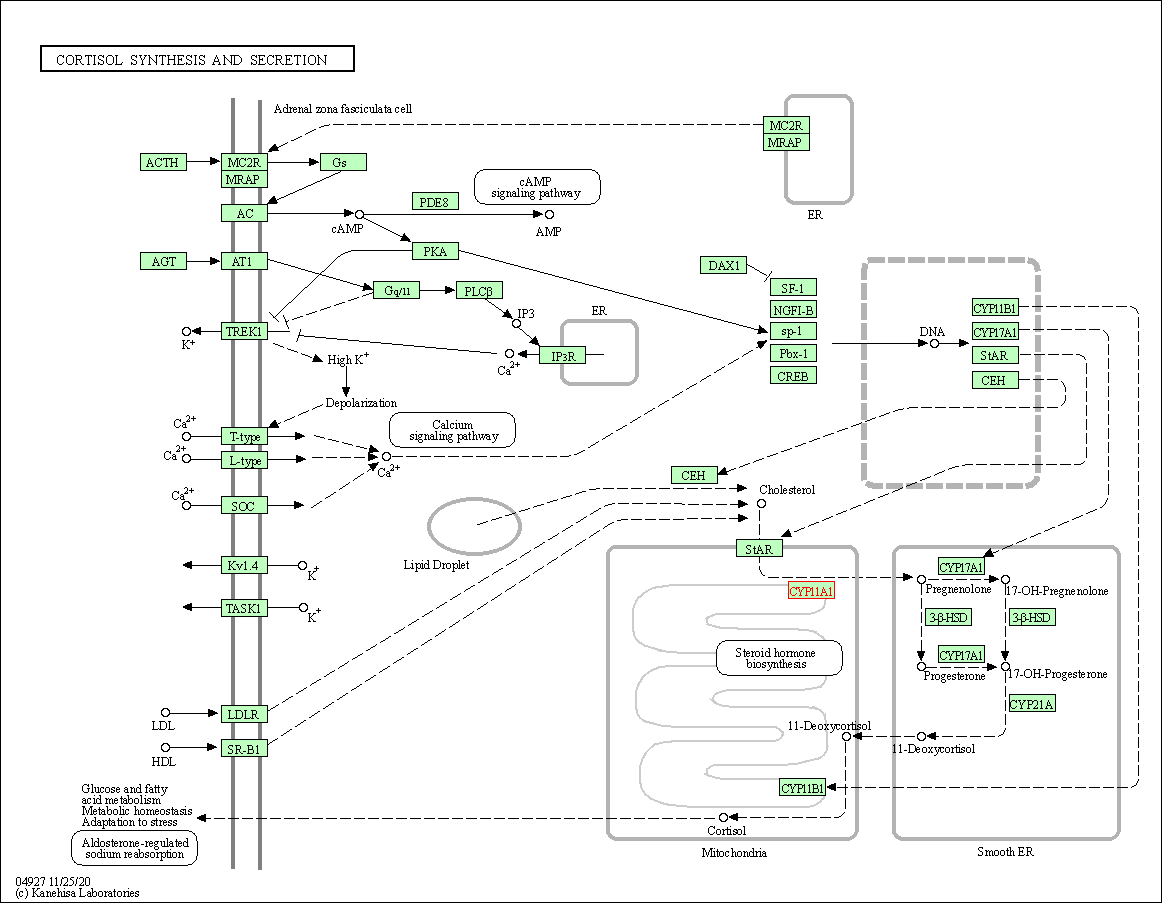
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Steroid hormone biosynthesis | hsa00140 | Affiliated Target |

|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
| Ovarian steroidogenesis | hsa04913 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Aldosterone synthesis and secretion | hsa04925 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Cortisol synthesis and secretion | hsa04927 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 6 | Degree centrality | 6.45E-04 | Betweenness centrality | 2.83E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 1.65E-01 | Radiality | 1.25E+01 | Clustering coefficient | 2.00E-01 |
| Neighborhood connectivity | 1.08E+01 | Topological coefficient | 3.05E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating Transcription Factors | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| BioCyc | [+] 2 BioCyc Pathways | + | ||||
| 1 | Superpathway of steroid hormone biosynthesis | |||||
| 2 | Pregnenolone biosynthesis | |||||
| KEGG Pathway | [+] 3 KEGG Pathways | + | ||||
| 1 | Steroid hormone biosynthesis | |||||
| 2 | Metabolic pathways | |||||
| 3 | Ovarian steroidogenesis | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | FSH Signaling Pathway | |||||
| Pathwhiz Pathway | [+] 1 Pathwhiz Pathways | + | ||||
| 1 | Steroidogenesis | |||||
| Reactome | [+] 1 Reactome Pathways | + | ||||
| 1 | Endogenous sterols | |||||
| WikiPathways | [+] 7 WikiPathways | + | ||||
| 1 | Prostaglandin Synthesis and Regulation | |||||
| 2 | Metapathway biotransformation | |||||
| 3 | Oxidation by Cytochrome P450 | |||||
| 4 | Metabolism of steroid hormones and vitamin D | |||||
| 5 | Glucocorticoid & Mineralcorticoid Metabolism | |||||
| 6 | Corticotropin-releasing hormone | |||||
| 7 | Phase 1 - Functionalization of compounds | |||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Breakdown of Th cell immune responses and steroidogenic CYP11A1 expression in CD4+ T cells in a murine model implanted with B16 melanoma. Cell Immunol. 2000 Nov 25;206(1):7-15. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7054). | |||||
| REF 3 | ClinicalTrials.gov (NCT03436485) Safety and Pharmacokinetics of ODM-208 in Patients With Metastatic Castration-resistant Prostate Cancer. U.S.National Institutes of Health. | |||||
| REF 4 | Clinical pipeline report, company report or official report of Merck | |||||
| REF 5 | Aromatase inhibitors. Synthesis and evaluation of mammary tumor inhibiting activity of 3-alkylated 3-(4-aminophenyl)piperidine-2,6-diones. J Med Chem. 1986 Aug;29(8):1362-9. | |||||
| REF 6 | Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc Natl Acad Sci U S A. 2011 Jun 21;108(25):10139-43. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

