Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T28692
(Former ID: TTDS00066)
|
|||||
| Target Name |
Lipoprotein lipase (LPL)
|
|||||
| Synonyms |
LIPD
Click to Show/Hide
|
|||||
| Gene Name |
LPL
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 1 Target-related Diseases | + | ||||
| 1 | Hyper-lipoproteinaemia [ICD-11: 5C80] | |||||
| Function |
Catalyzes the hydrolysis of triglycerides from circulating chylomicrons and very low density lipoproteins (VLDL), and thereby plays an important role in lipid clearance from the blood stream, lipid utilization and storage. Mediates margination of triglyceride-rich lipoprotein particles in capillaries. Recruited to its site of action on the luminal surface of vascular endothelium by binding to GPIHBP1 and cell surface heparan sulfate proteoglycans. Key enzyme in triglyceride metabolism.
Click to Show/Hide
|
|||||
| BioChemical Class |
Carboxylic ester hydrolase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.1.1.34
|
|||||
| Sequence |
MESKALLVLTLAVWLQSLTASRGGVAAADQRRDFIDIESKFALRTPEDTAEDTCHLIPGV
AESVATCHFNHSSKTFMVIHGWTVTGMYESWVPKLVAALYKREPDSNVIVVDWLSRAQEH YPVSAGYTKLVGQDVARFINWMEEEFNYPLDNVHLLGYSLGAHAAGIAGSLTNKKVNRIT GLDPAGPNFEYAEAPSRLSPDDADFVDVLHTFTRGSPGRSIGIQKPVGHVDIYPNGGTFQ PGCNIGEAIRVIAERGLGDVDQLVKCSHERSIHLFIDSLLNEENPSKAYRCSSKEAFEKG LCLSCRKNRCNNLGYEINKVRAKRSSKMYLKTRSQMPYKVFHYQVKIHFSGTESETHTNQ AFEISLYGTVAESENIPFTLPEVSTNKTYSFLIYTEVDIGELLMLKLKWKSDSYFSWSDW WSSPGFAIQKIRVKAGETQKKVIFCSREKVSHLQKGKAPAVFVKCHDKSLNKKSG Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A01285 ; BADD_A02260 ; BADD_A02367 ; BADD_A03749 ; BADD_A04520 ; BADD_A05675 | |||||
| HIT2.0 ID | T99EYH | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 2 Approved Drugs | + | ||||
| 1 | Clofibrate | Drug Info | Approved | Dysbetalipoproteinemia | [2], [3] | |
| 2 | Gemfibrozil | Drug Info | Approved | Hyperlipidaemia | [4], [5] | |
| Clinical Trial Drug(s) | [+] 2 Clinical Trial Drugs | + | ||||
| 1 | AMT-011 | Drug Info | Phase 3 | Blood forming organ disorder | [6] | |
| 2 | Ibrolipim | Drug Info | Phase 2 | Hyperlipidaemia | [7] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Activator | [+] 2 Activator drugs | + | ||||
| 1 | Clofibrate | Drug Info | [1] | |||
| 2 | Gemfibrozil | Drug Info | [8] | |||
| Modulator | [+] 3 Modulator drugs | + | ||||
| 1 | AMT-011 | Drug Info | [6] | |||
| 2 | Ibrolipim | Drug Info | [6], [7] | |||
| 3 | WS-070117 | Drug Info | [6] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: 7-(3-cyano-4-hydroxyphenyl)-N-[2-(morpholin-4-yl)ethyl]dibenzo[b,f]oxepine-10-carboxamide | Ligand Info | |||||
| Structure Description | Compound 2 bound structure of WT Lipoprotein Lipase in Complex with GPIHBP1 Mutant N78D N82D produced in HEK293-F cells | PDB:6OB0 | ||||
| Method | X-ray diffraction | Resolution | 2.81 Å | Mutation | Yes | [9] |
| PDB Sequence |
QRRDFIDIES
39 KFALRTPEDT49 AEDTCHLIPG59 VAESVATCHF69 NHSSKTFMVI79 HGWTVTGMYE 89 SWVPKLVAAL99 YKREPDSNVI109 VVDWLSRAQE119 HYPVSAGYTK129 LVGQDVARFI 139 NWMEEEFNYP149 LDNVHLLGYS159 LGAHAAGIAG169 SLTNKKVNRI179 TGLDPAGPNF 189 EYAEAPSRLS199 PDDADFVDVL209 HTFTRGSPGR219 SIGIQKPVGH229 VDIYPNGGTF 239 QPGCNIGEAI249 RVIAERGLGD259 VDQLVKCSHE269 RSIHLFIDSL279 LNEENPSKAY 289 RCSSKEAFEK299 GLCLSCRKNR309 CNNLGYEINK319 VRAKRSSKMY329 LKTRSQMPYK 339 VFHYQVKIHF349 SGTESETHTN359 QAFEISLYGT369 VAESENIPFT379 LPEVSTNKTY 389 SFLIYTEVDI399 GELLMLKLKW409 KSDSYFSWSD419 WWSSPGFAIQ429 KIRVKAGETQ 439 KKVIFCSREK449 VSHLQKGKAP459 AVFVKCHDKS469 LN
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
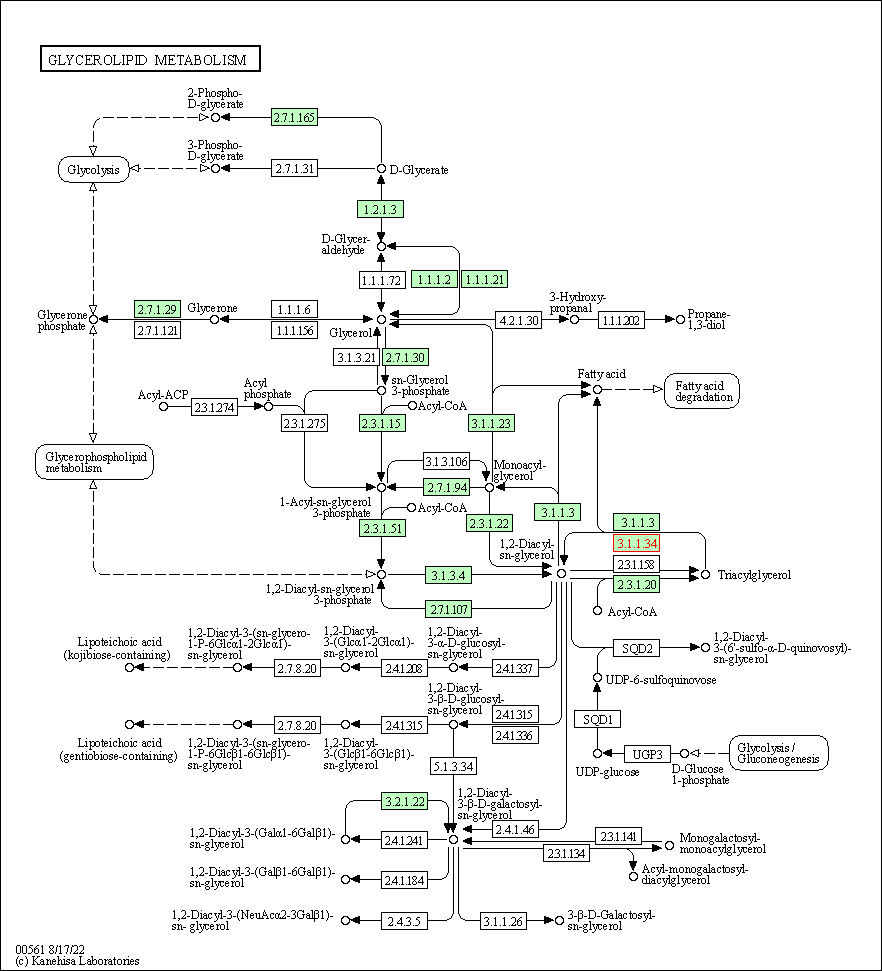
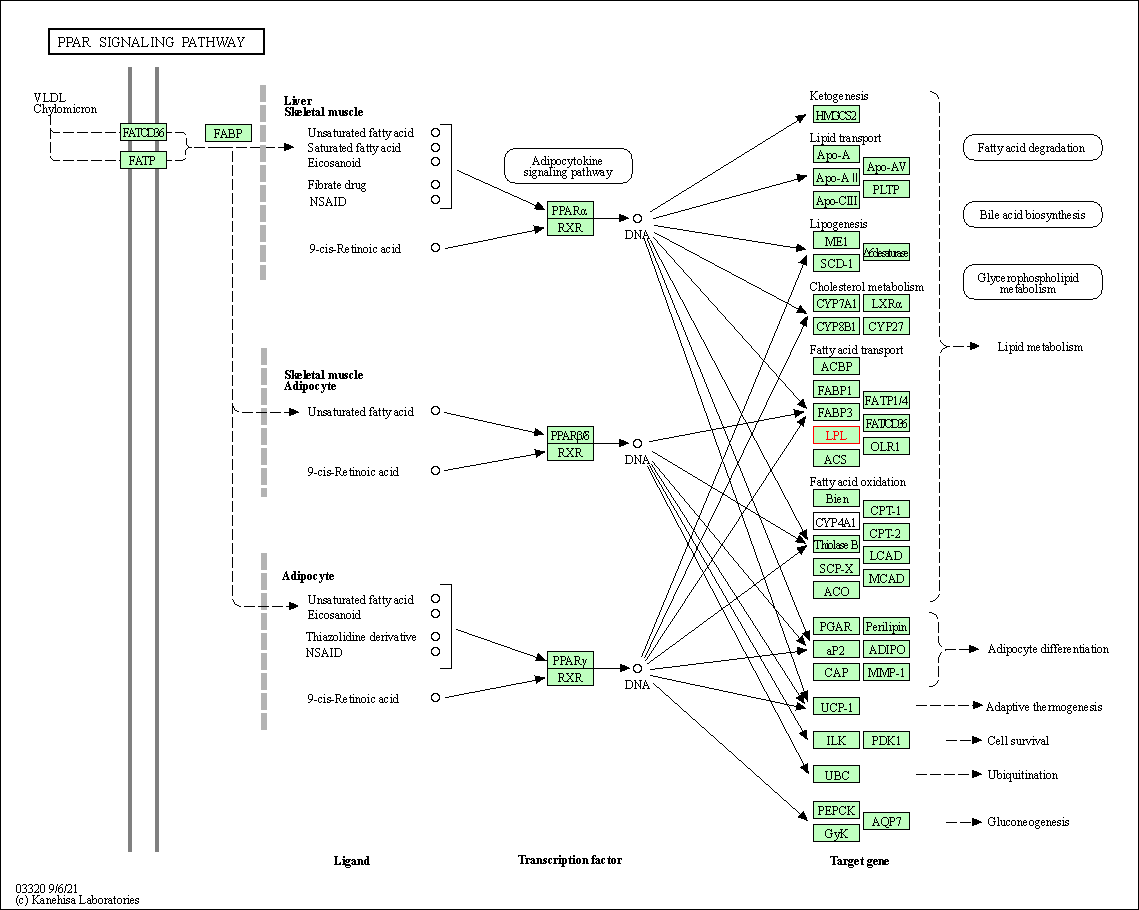
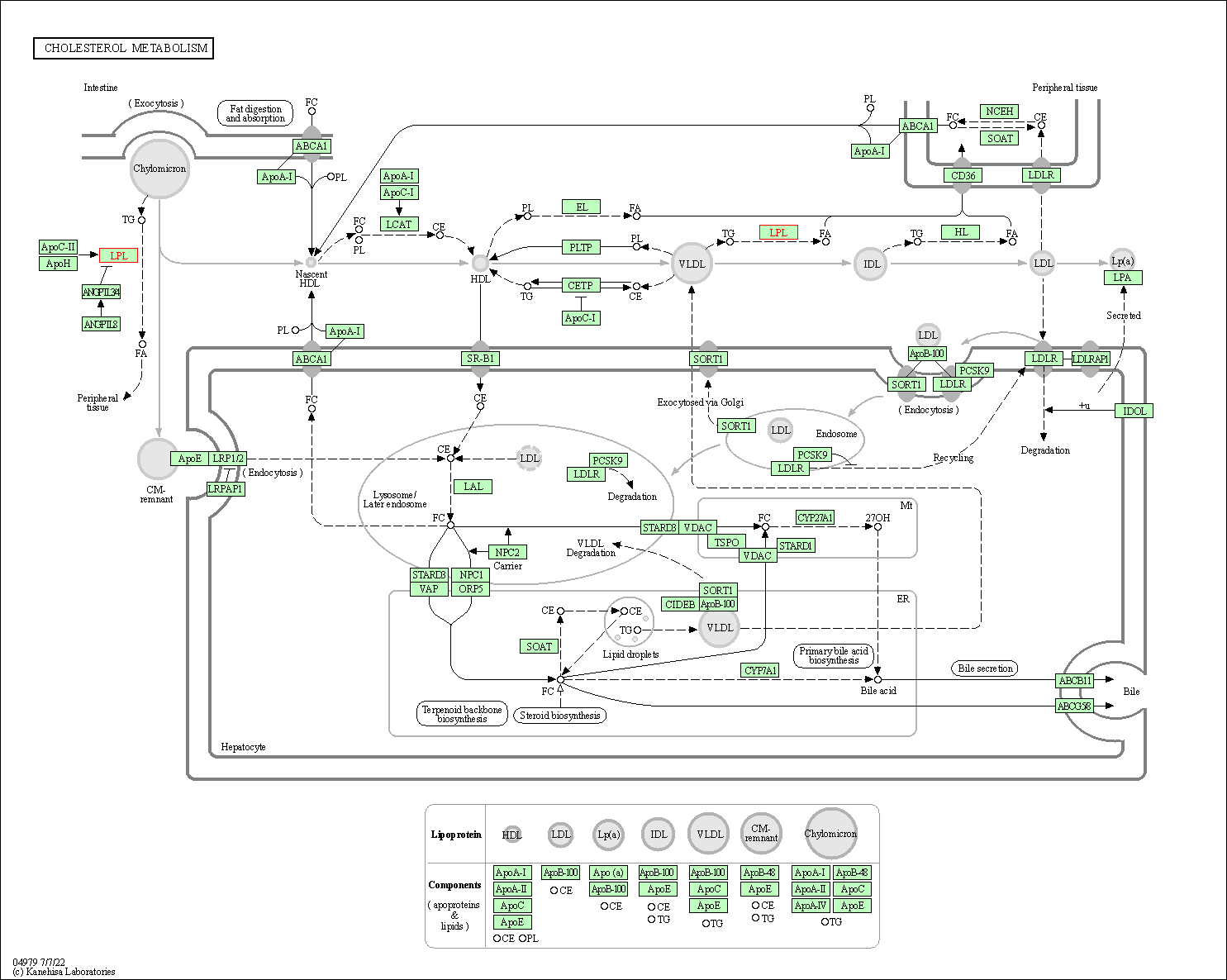
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Glycerolipid metabolism | hsa00561 | Affiliated Target |

|
| Class: Metabolism => Lipid metabolism | Pathway Hierarchy | ||
| PPAR signaling pathway | hsa03320 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Cholesterol metabolism | hsa04979 | Affiliated Target |

|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Degree | 20 | Degree centrality | 2.15E-03 | Betweenness centrality | 1.58E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.23E-01 | Radiality | 1.39E+01 | Clustering coefficient | 2.58E-01 |
| Neighborhood connectivity | 2.05E+01 | Topological coefficient | 8.91E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-regulating Transcription Factors | ||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Effects of clofibrate treatment in laying hens. Poult Sci. 2007 Jun;86(6):1187-95. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2667). | |||||
| REF 3 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 070531. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3439). | |||||
| REF 5 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 074256. | |||||
| REF 6 | Alipogene tiparvovec for the treatment of lipoprotein lipase deficiency. Drugs Today (Barc). 2013 Mar;49(3):161-70. | |||||
| REF 7 | NO-1886 (ibrolipim), a lipoprotein lipase activator, increases the expression of uncoupling protein 3 in skeletal muscle and suppresses fat accumulation in high-fat diet-induced obesity in rats. Metabolism. 2005 Dec;54(12):1587-92. | |||||
| REF 8 | Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today (Barc). 2006 Jan;42(1):39-64. | |||||
| REF 9 | Structure of lipoprotein lipase in complex with GPIHBP1. Proc Natl Acad Sci U S A. 2019 May 21;116(21):10360-10365. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

