Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T34234
(Former ID: TTDS00251)
|
|||||
| Target Name |
Vitamin D3 receptor (VDR)
|
|||||
| Synonyms |
Vitamin D(3) receptor; Nuclear vitamin D receptor; Nuclear receptor subfamily 1 group I member 1; NR1I1; 1,25-dihydroxyvitamin D3 receptor
Click to Show/Hide
|
|||||
| Gene Name |
VDR
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 7 Target-related Diseases | + | ||||
| 1 | Chronic kidney disease [ICD-11: GB61] | |||||
| 2 | Hair/hair growth developmental defect [ICD-11: LC30] | |||||
| 3 | Hyper-parathyroidism [ICD-11: 5A51] | |||||
| 4 | Hypo-parathyroidism [ICD-11: 5A50] | |||||
| 5 | Mineral deficiency [ICD-11: 5B5K] | |||||
| 6 | Psoriasis [ICD-11: EA90] | |||||
| 7 | Vitamin deficiency [ICD-11: 5B55-5B5F] | |||||
| Function |
Enters the nucleus upon vitamin D3 binding where it forms heterodimers with the retinoid X receptor/RXR. The VDR-RXR heterodimers bind to specific response elements on DNA and activate the transcription of vitamin D3-responsive target genes. Plays a central role in calcium homeostasis. Nuclear receptor for calcitriol, the active form of vitamin D3 which mediates the action of this vitamin on cells.
Click to Show/Hide
|
|||||
| BioChemical Class |
Nuclear hormone receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MEAMAASTSLPDPGDFDRNVPRICGVCGDRATGFHFNAMTCEGCKGFFRRSMKRKALFTC
PFNGDCRITKDNRRHCQACRLKRCVDIGMMKEFILTDEEVQRKREMILKRKEEEALKDSL RPKLSEEQQRIIAILLDAHHKTYDPTYSDFCQFRPPVRVNDGGGSHPSRPNSRHTPSFSG DSSSSCSDHCITSSDMMDSSSFSNLDLSEEDSDDPSVTLELSQLSMLPHLADLVSYSIQK VIGFAKMIPGFRDLTSEDQIVLLKSSAIEVIMLRSNESFTMDDMSWTCGNQDYKYRVSDV TKAGHSLELIEPLIKFQVGLKKLNLHEEEHVLLMAICIVSPDRPGVQDAALIEAIQDRLS NTLQTYIRCRHPPPGSHLLYAKMIQKLADLRSLNEEHSKQYRCLSFQPECSMKLTPLVLE VFGNEIS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T85JI5 | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 9 Approved Drugs | + | ||||
| 1 | Calcidiol | Drug Info | Approved | Vitamin D deficiency | [2], [3] | |
| 2 | Calcipotriol | Drug Info | Approved | Psoriasis vulgaris | [4], [5] | |
| 3 | Calcitriol | Drug Info | Approved | Congenital alopecia | [6], [7] | |
| 4 | Cholecalciferol | Drug Info | Approved | Vitamin D deficiency | [8], [9] | |
| 5 | Dihydrotachysterol | Drug Info | Approved | Hypocalcemia | [10] | |
| 6 | Doxercalciferol | Drug Info | Approved | Chronic kidney disease | [11], [12] | |
| 7 | Ergocalciferol | Drug Info | Approved | Hypoparathyroidism | [13] | |
| 8 | Falecalcitrol | Drug Info | Approved | Hyperparathyroidism | [10] | |
| 9 | Paricalcitol | Drug Info | Approved | Hyperparathyroidism | [5], [14] | |
| Clinical Trial Drug(s) | [+] 5 Clinical Trial Drugs | + | ||||
| 1 | S-06911 | Drug Info | Phase 3 | Osteoporosis | [15] | |
| 2 | Seocalcitol | Drug Info | Phase 3 | Rickets | [16], [17] | |
| 3 | Inecalcitol oral | Drug Info | Phase 2 | Prostate cancer | [18], [19] | |
| 4 | CTAP-201 | Drug Info | Phase 1 | Hyperparathyroidism | [20] | |
| 5 | RO-23-7553 | Drug Info | Phase 1 | Prostate disease | [21] | |
| Discontinued Drug(s) | [+] 8 Discontinued Drugs | + | ||||
| 1 | Atocalcitol | Drug Info | Discontinued in Phase 2 | Psoriasis vulgaris | [22] | |
| 2 | Lexacalcitol | Drug Info | Discontinued in Phase 2 | Solid tumour/cancer | [23], [24] | |
| 3 | RO-26-9228 | Drug Info | Discontinued in Phase 2 | Urinary incontinence | [25], [26] | |
| 4 | Tisocalcitate | Drug Info | Discontinued in Phase 2 | Psoriasis vulgaris | [27] | |
| 5 | BXL-746 | Drug Info | Discontinued in Phase 1 | Inflammation | [28] | |
| 6 | CB-1267 | Drug Info | Terminated | Prostate cancer | [29] | |
| 7 | CEP-4186 | Drug Info | Terminated | Acute myeloid leukaemia | [30] | |
| 8 | Ecalcidene | Drug Info | Terminated | Acne vulgaris | [31] | |
| Mode of Action | [+] 6 Modes of Action | + | ||||
| Antagonist | [+] 7 Antagonist drugs | + | ||||
| 1 | Calcidiol | Drug Info | [1], [32] | |||
| 2 | Calcipotriol | Drug Info | [33] | |||
| 3 | Dihydrotachysterol | Drug Info | [37], [32] | |||
| 4 | Doxercalciferol | Drug Info | [12] | |||
| 5 | Ergocalciferol | Drug Info | [1], [32] | |||
| 6 | TEI-9647 | Drug Info | [60] | |||
| 7 | ZK159222 | Drug Info | [61] | |||
| Agonist | [+] 26 Agonist drugs | + | ||||
| 1 | Calcitriol | Drug Info | [34] | |||
| 2 | Falecalcitrol | Drug Info | [38] | |||
| 3 | Paricalcitol | Drug Info | [39] | |||
| 4 | Inecalcitol oral | Drug Info | [19], [42] | |||
| 5 | CTAP-201 | Drug Info | [43] | |||
| 6 | RO-23-7553 | Drug Info | [44], [10] | |||
| 7 | PMID27454349-Compound-100 | Drug Info | [45] | |||
| 8 | PMID27454349-Compound-101 | Drug Info | [45] | |||
| 9 | PMID27454349-Compound-102 | Drug Info | [45] | |||
| 10 | PMID27454349-Compound-91 | Drug Info | [45] | |||
| 11 | PMID27454349-Compound-92 | Drug Info | [45] | |||
| 12 | PMID27454349-Compound-93 | Drug Info | [45] | |||
| 13 | PMID27454349-Compound-94 | Drug Info | [45] | |||
| 14 | PMID27454349-Compound-95 | Drug Info | [45] | |||
| 15 | PMID27454349-Compound-96 | Drug Info | [45] | |||
| 16 | PMID27454349-Compound-97 | Drug Info | [45] | |||
| 17 | PMID27454349-Compound-98 | Drug Info | [45] | |||
| 18 | PMID27454349-Compound-99 | Drug Info | [45] | |||
| 19 | Atocalcitol | Drug Info | [46] | |||
| 20 | Lexacalcitol | Drug Info | [47] | |||
| 21 | RO-26-9228 | Drug Info | [40] | |||
| 22 | 2MD | Drug Info | [53] | |||
| 23 | 3-keto-lithocholic acid | Drug Info | [54] | |||
| 24 | calcitriol-26,23-lactone | Drug Info | [55] | |||
| 25 | gemini | Drug Info | [56] | |||
| 26 | LG190178 | Drug Info | [58] | |||
| Binder | [+] 1 Binder drugs | + | ||||
| 1 | Cholecalciferol | Drug Info | [32], [35], [36] | |||
| Inhibitor | [+] 5 Inhibitor drugs | + | ||||
| 1 | S-06911 | Drug Info | [40] | |||
| 2 | Seocalcitol | Drug Info | [41] | |||
| 3 | Tisocalcitate | Drug Info | [48] | |||
| 4 | KSP-BCS-1-alpha-CHF2-1624F2-2 | Drug Info | [57] | |||
| 5 | KSP-BCS-1-alpha-CHF2-20-epi-22-oxabishomo-26-OH | Drug Info | [57] | |||
| Stimulator | [+] 1 Stimulator drugs | + | ||||
| 1 | BXL-746 | Drug Info | [49] | |||
| Modulator | [+] 4 Modulator drugs | + | ||||
| 1 | CB-1267 | Drug Info | [50] | |||
| 2 | CEP-4186 | Drug Info | [51] | |||
| 3 | Ecalcidene | Drug Info | [52] | |||
| 4 | MC-1301 | Drug Info | [59] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Calcipotriol | Ligand Info | |||||
| Structure Description | Crystal structure of VDR ligand binding domain complexed to calcipotriol. | PDB:1S19 | ||||
| Method | X-ray diffraction | Resolution | 2.10 Å | Mutation | No | [62] |
| PDB Sequence |
LRPKLSEEQQ
129 RIIAILLDAH139 HKTYDPTYSD149 FCQFRPPVRV159 NDGGGSVTLE220 LSQLSMLPHL 230 ADLVSYSIQK240 VIGFAKMIPG250 FRDLTSEDQI260 VLLKSSAIEV270 IMLRSNESFT 280 MDDMSWTCGN290 QDYKYRVSDV300 TKAGHSLELI310 EPLIKFQVGL320 KKLNLHEEEH 330 VLLMAICIVS340 PDRPGVQDAA350 LIEAIQDRLS360 NTLQTYIRCR370 HPPPGSHLLY 380 AKMIQKLADL390 RSLNEEHSKQ400 YRCLSFQPEC410 SMKLTPLVLE420 VFG |
|||||
|
|
TYR143
2.768
TYR147
3.839
PHE150
4.197
LEU227
3.503
LEU230
4.052
ALA231
3.953
LEU233
3.665
VAL234
3.649
SER237
2.791
ILE268
4.437
ILE271
3.740
MET272
4.230
ARG274
2.839
SER275
3.406
|
|||||
| Ligand Name: Calcitriol | Ligand Info | |||||
| Structure Description | Crystal Structure Of The Nuclear Receptor For Vitamin D Ligand Binding Domain Bound to MC1288 | PDB:1IE9 | ||||
| Method | X-ray diffraction | Resolution | 1.40 Å | Mutation | No | [63] |
| PDB Sequence |
DSLRPKLSEE
127 QQRIIAILLD137 AHHKTYDPTY147 SDFCQFRPPV157 RVNDGGGSVT218 LELSQLSMLP 228 HLADLVSYSI238 QKVIGFAKMI248 PGFRDLTSED258 QIVLLKSSAI268 EVIMLRSNES 278 FTMDDMSWTC288 GNQDYKYRVS298 DVTKAGHSLE308 LIEPLIKFQV318 GLKKLNLHEE 328 EHVLLMAICI338 VSPDRPGVQD348 AALIEAIQDR358 LSNTLQTYIR368 CRHPPPGSHL 378 LYAKMIQKLA388 DLRSLNEEHS398 KQYRCLSFQP408 ECSMKLTPLV418 LEVFG |
|||||
|
|
TYR143
2.776
TYR147
3.661
PHE150
4.136
LEU227
3.418
LEU230
3.885
LEU233
3.605
VAL234
3.598
SER237
2.809
ILE268
4.206
ILE271
3.883
MET272
4.137
ARG274
2.949
SER275
3.416
SER278
2.872
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
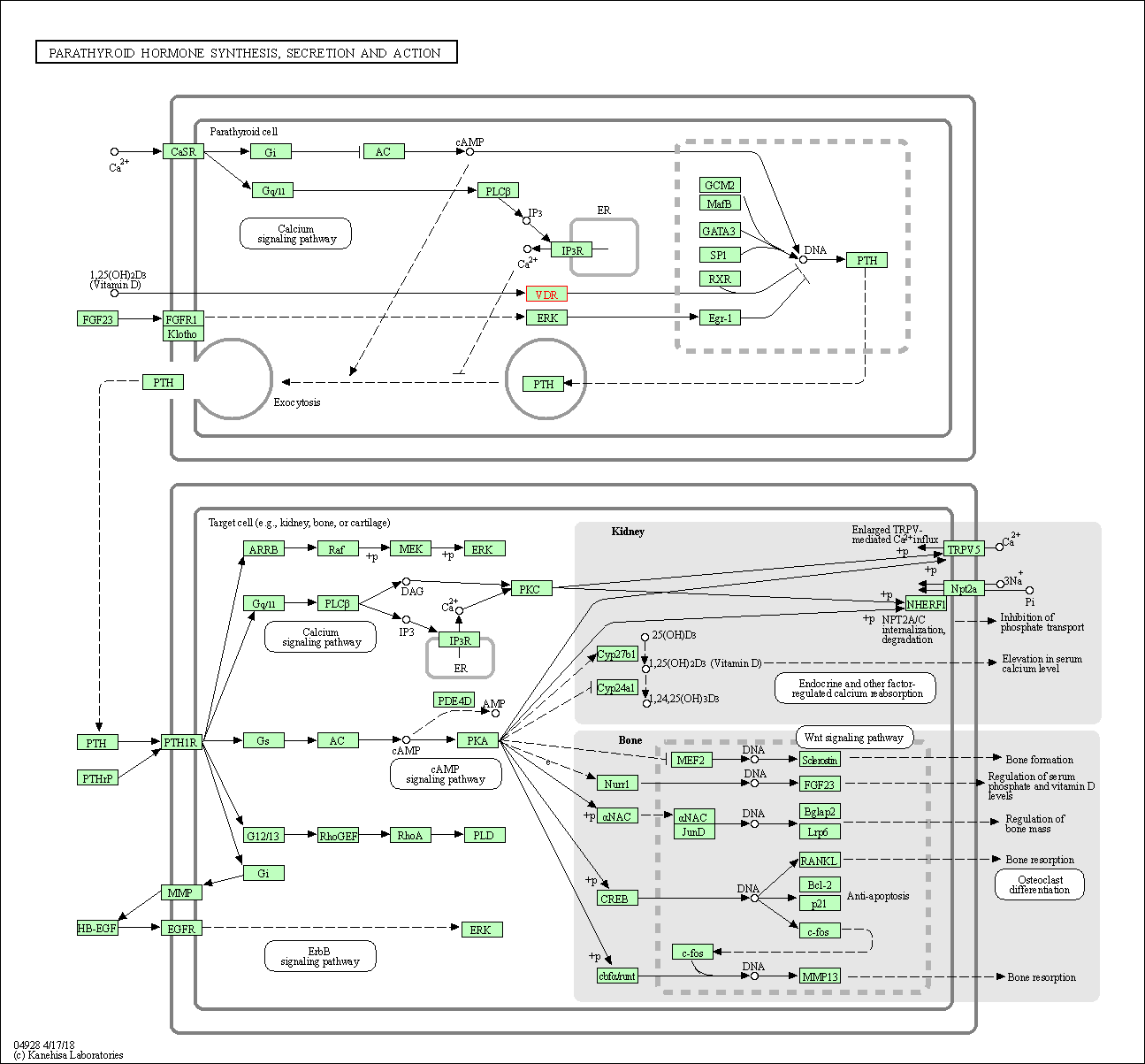
| Parathyroid hormone synthesis, secretion and action | hsa04928 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
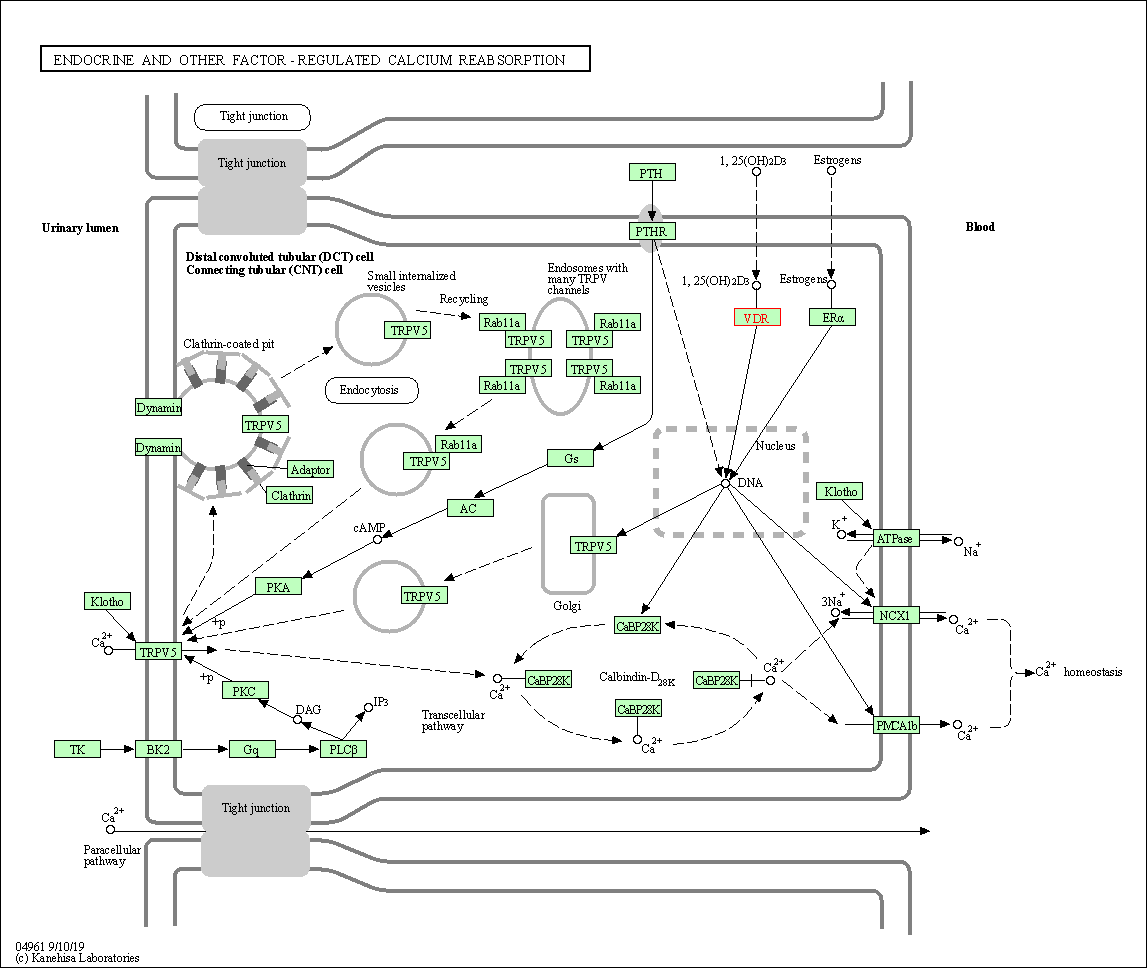
| Endocrine and other factor-regulated calcium reabsorption | hsa04961 | Affiliated Target |

|
| Class: Organismal Systems => Excretory system | Pathway Hierarchy | ||
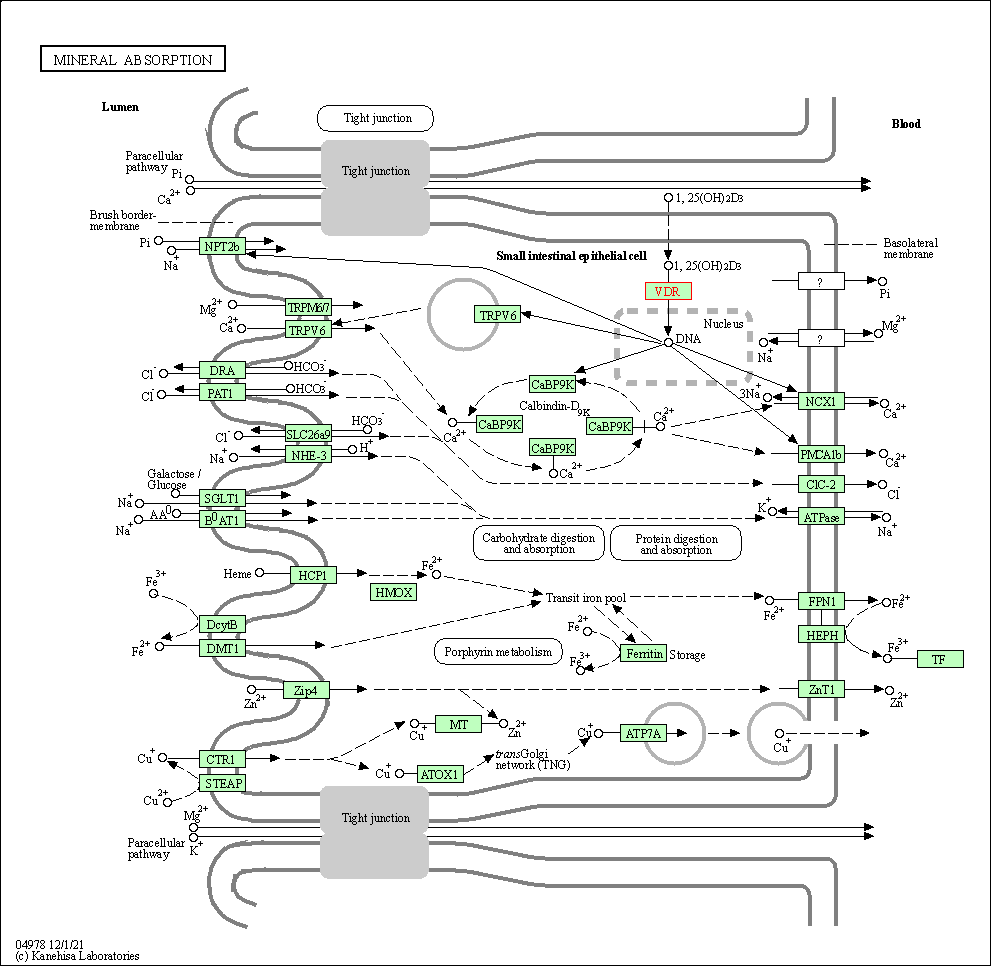
| Mineral absorption | hsa04978 | Affiliated Target |

|
| Class: Organismal Systems => Digestive system | Pathway Hierarchy | ||
| Degree | 16 | Degree centrality | 1.72E-03 | Betweenness centrality | 9.10E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.39E-01 | Radiality | 1.42E+01 | Clustering coefficient | 3.17E-01 |
| Neighborhood connectivity | 5.50E+01 | Topological coefficient | 1.10E-01 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 3 KEGG Pathways | + | ||||
| 1 | Endocrine and other factor-regulated calcium reabsorption | |||||
| 2 | Mineral absorption | |||||
| 3 | Tuberculosis | |||||
| NetPath Pathway | [+] 1 NetPath Pathways | + | ||||
| 1 | IL4 Signaling Pathway | |||||
| Panther Pathway | [+] 1 Panther Pathways | + | ||||
| 1 | Vitamin D metabolism and pathway | |||||
| PID Pathway | [+] 6 PID Pathways | + | ||||
| 1 | Regulation of nuclear SMAD2/3 signaling | |||||
| 2 | Direct p53 effectors | |||||
| 3 | RXR and RAR heterodimerization with other nuclear receptor | |||||
| 4 | Retinoic acid receptors-mediated signaling | |||||
| 5 | Validated transcriptional targets of deltaNp63 isoforms | |||||
| 6 | Validated transcriptional targets of TAp63 isoforms | |||||
| Reactome | [+] 1 Reactome Pathways | + | ||||
| 1 | Nuclear Receptor transcription pathway | |||||
| WikiPathways | [+] 7 WikiPathways | + | ||||
| 1 | Ovarian Infertility Genes | |||||
| 2 | Nuclear Receptors in Lipid Metabolism and Toxicity | |||||
| 3 | Nuclear Receptors Meta-Pathway | |||||
| 4 | Vitamin D Receptor Pathway | |||||
| 5 | Drug Induction of Bile Acid Pathway | |||||
| 6 | Nuclear Receptors | |||||
| 7 | Vitamin D Metabolism | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008 Sep;3(5):1535-41. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6921). | |||||
| REF 3 | Drug Information of Carbetocin from nextbio research in illumina. 2015. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2778). | |||||
| REF 5 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2779). | |||||
| REF 7 | ClinicalTrials.gov (NCT02186665) Plaque Psoriasis Study in Pediatric Subjects. U.S. National Institutes of Health. | |||||
| REF 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2747). | |||||
| REF 9 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 021762. | |||||
| REF 10 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2790). | |||||
| REF 12 | Emerging drugs for psoriasis. Expert Opin Emerg Drugs. 2009 Mar;14(1):145-63. | |||||
| REF 13 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 040833. | |||||
| REF 14 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2791). | |||||
| REF 15 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 700201063) | |||||
| REF 16 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2777). | |||||
| REF 17 | Seocalcitol (EB 1089): a vitamin D analogue of anti-cancer potential. Background, design, synthesis, pre-clinical and clinical evaluation. Curr Pharm Des. 2000 May;6(7):803-28. | |||||
| REF 18 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7747). | |||||
| REF 19 | Inecalcitol, an analog of 1,25D3, displays enhanced antitumor activity through the induction of apoptosis in a squamous cell carcinoma model system. Cell Cycle. 2013 Mar 1;12(5):743-52. | |||||
| REF 20 | ClinicalTrials.gov (NCT00792857) Comparison of I.V. CTAP201 and Doxercalciferol (Hectorol) in Subjects With Chronic Kidney Disease (CKD) and Secondary Hyperparathyroidism (SHPT). U.S. National Institutes of Health. | |||||
| REF 21 | ClinicalTrials.gov (NCT00004926) ILX23-7553 in Treating Patients With Solid Tumors That Have Not Responded to Previous Therapy. U.S. National Institutes of Health. | |||||
| REF 22 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800014844) | |||||
| REF 23 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2775). | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800002985) | |||||
| REF 25 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2785). | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800015074) | |||||
| REF 27 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018814) | |||||
| REF 28 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025073) | |||||
| REF 29 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800007891) | |||||
| REF 30 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800012493) | |||||
| REF 31 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800019289) | |||||
| REF 32 | Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1689S-96S. | |||||
| REF 33 | Cellular and molecular mechanisms involved in the action of vitamin D analogs targeting vitiligo depigmentation. Curr Drug Targets. 2008 Apr;9(4):345-59. | |||||
| REF 34 | Kaposi sarcoma is a therapeutic target for vitamin D(3) receptor agonist. Blood. 2000 Nov 1;96(9):3188-94. | |||||
| REF 35 | [Vitamin D2 or vitamin D3]. Rev Med Interne. 2008 Oct;29(10):815-20. | |||||
| REF 36 | Genetic polymorphisms in vitamin D receptor, vitamin D-binding protein, Toll-like receptor 2, nitric oxide synthase 2, and interferon-gamma genes and its association with susceptibility to tuberculosis. Braz J Med Biol Res. 2009 Apr;42(4):312-22. | |||||
| REF 37 | In vivo metabolism of the vitamin D analog, dihydrotachysterol. Evidence for formation of 1 alpha,25- and 1 beta,25-dihydroxy-dihydrotachysterol metabolites and studies of their biological activity. J Biol Chem. 1993 Jan 5;268(1):282-92. | |||||
| REF 38 | Synthesis and biological evaluations of A-ring isomers of 26,26,26,27,27,27-hexafluoro-1,25-dihydroxyvitamin D3. Bioorg Med Chem. 2000 Aug;8(8):2157-66. | |||||
| REF 39 | New acquisitions in therapy of secondary hyperparathyroidism in chronic kidney disease and peritoneal dialysis patients: role of vitamin D receptor... Contrib Nephrol. 2009;163:219-226. | |||||
| REF 40 | Evidence for tissue- and cell-type selective activation of the vitamin D receptor by Ro-26-9228, a noncalcemic analog of vitamin D3. J Cell Biochem. 2003 Feb 1;88(2):267-73. | |||||
| REF 41 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 42 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 43 | Vascular calcification and secondary hyperparathyroidism of severe chronic kidney disease and its relation to serum phosphate and calcium levels. Br J Pharmacol. 2009 April; 156(8): 1267-1278. | |||||
| REF 44 | Three synthetic vitamin D analogues induce prostate-specific acid phosphatase and prostate-specific antigen while inhibiting the growth of human prostate cancer cells in a vitamin D receptor-dependent fashion. Clin Cancer Res. 1997 Aug;3(8):1331-8. | |||||
| REF 45 | Vitamin D receptor 2016: novel ligands and structural insights.Expert Opin Ther Pat. 2016 Nov;26(11):1291-1306. | |||||
| REF 46 | Elocalcitol, a vitamin D3 analog for the potential treatment of benign prostatic hyperplasia, overactive bladder and male infertility. IDrugs. 2009 Jun;12(6):381-93. | |||||
| REF 47 | The vitamin D analog, KH1060, is rapidly degraded both in vivo and in vitro via several pathways: principal metabolites generated retain significant biological activity. Endocrinology. 1997 Dec;138(12):5485-96. | |||||
| REF 48 | The Rationale Behind Topical Vitamin D Analogs in the Treatment of Psoriasis: Where Does Topical Calcitriol Fit In . J Clin Aesthet Dermatol. 2010 August; 3(8): 46-53. | |||||
| REF 49 | Inter-species differences in sensitivity to the calcemic activity of the novel 1,25-dihydroxyvitamin D3 analog BXL746. Regul Toxicol Pharmacol. 2008 Dec;52(3):332-41. | |||||
| REF 50 | Novel 20-epi-vitamin D3 analog combined with 9-cis-retinoic acid markedly inhibits colony growth of prostate cancer cells. Prostate. 1999 Aug 1;40(3):141-9. | |||||
| REF 51 | Novel vitamin D3 analog (CB1093) when combined with paclitaxel and cisplatin inhibit growth of MCF-7 human breast cancer cells in vivo. Int J Oncol. 1998 Sep;13(3):421-8. | |||||
| REF 52 | Degradation chemistry of a Vitamin D analogue (ecalcidene) investigated by HPLC-MS, HPLC-NMR and chemical derivatization. J Pharm Biomed Anal. 2006 Mar 3;40(4):850-63. | |||||
| REF 53 | New 1alpha,25-dihydroxy-19-norvitamin D3 compounds of high biological activity: synthesis and biological evaluation of 2-hydroxymethyl, 2-methyl, and 2-methylene analogues. J Med Chem. 1998 Nov 5;41(23):4662-74. | |||||
| REF 54 | Vitamin D receptor as an intestinal bile acid sensor. Science. 2002 May 17;296(5571):1313-6. | |||||
| REF 55 | Biological activity of 24,24-difluoro-1 alpha, 25-dihydroxyvitamin D3 and 1 alpha, 25-dihydroxyvitamin D3-26,23-lactone in inducing differentiation of human myeloid leukemia cells. Arch Biochem Biophys. 1983 Jan;220(1):90-4. | |||||
| REF 56 | Novel Gemini analogs of 1alpha,25-dihydroxyvitamin D(3) with enhanced transcriptional activity. Biochem Pharmacol. 2004 Apr 1;67(7):1327-36. | |||||
| REF 57 | Low-calcemic, highly antiproliferative, 1-difluoromethyl hybrid analogs of the natural hormone 1alpha,25-dihydroxyvitamin D3: design, synthesis, an... J Med Chem. 2006 Dec 14;49(25):7513-7. | |||||
| REF 58 | Novel nonsecosteroidal vitamin D mimics exert VDR-modulating activities with less calcium mobilization than 1,25-dihydroxyvitamin D3. Chem Biol. 1999 May;6(5):265-75. | |||||
| REF 59 | Effects of 1,25-dihydroxyvitamin D3 and its 20-epi analogues (MC 1288, MC 1301, KH 1060), on clonal keratinocyte growth: evidence for differentiati... Br J Pharmacol. 1997 Mar;120(6):1119-27. | |||||
| REF 60 | Further synthetic and biological studies on vitamin D hormone antagonists based on C24-alkylation and C2alpha-functionalization of 25-dehydro-1alpha-hydroxyvitamin D(3)-26,23-lactones. J Med Chem. 2006 Nov 30;49(24):7063-75. | |||||
| REF 61 | Carboxylic ester antagonists of 1alpha,25-dihydroxyvitamin D(3) show cell-specific actions. Chem Biol. 2000 Nov;7(11):885-94. | |||||
| REF 62 | Crystal structures of the vitamin D nuclear receptor liganded with the vitamin D side chain analogues calcipotriol and seocalcitol, receptor agonists of clinical importance. Insights into a structural basis for the switching of calcipotriol to a receptor antagonist by further side chain modification. J Med Chem. 2004 Apr 8;47(8):1956-61. | |||||
| REF 63 | Crystal structures of the vitamin D receptor complexed to superagonist 20-epi ligands. Proc Natl Acad Sci U S A. 2001 May 8;98(10):5491-6. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

