Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T58921
(Former ID: TTDS00338)
|
|||||
| Target Name |
Peroxisome proliferator-activated receptor gamma (PPAR-gamma)
|
|||||
| Synonyms |
PPAR-gamma; Nuclear receptor subfamily 1 group C member 3; NR1C3
Click to Show/Hide
|
|||||
| Gene Name |
PPARG
|
|||||
| Target Type |
Successful target
|
[1] | ||||
| Disease | [+] 3 Target-related Diseases | + | ||||
| 1 | Acute diabete complication [ICD-11: 5A2Y] | |||||
| 2 | Alzheimer disease [ICD-11: 8A20] | |||||
| 3 | Type 2 diabetes mellitus [ICD-11: 5A11] | |||||
| Function |
Nuclear receptor that binds peroxisome proliferators such as hypolipidemic drugs and fatty acids. Once activated by a ligand, the nuclear receptor binds to DNA specific PPAR response elements (PPRE) and modulates the transcription of its target genes, such as acyl-CoA oxidase. It therefore controls the peroxisomal beta-oxidation pathway of fatty acids. Key regulator of adipocyte differentiation and glucose homeostasis. ARF6 acts as a key regulator of the tissue-specific adipocyte P2 (aP2) enhancer. Acts as a critical regulator of gut homeostasis by suppressing NF-kappa-B-mediated proinflammatory responses. Plays a role in the regulation of cardiovascular circadian rhythms by regulating the transcription of ARNTL/BMAL1 in the blood vessels (By similarity).
Click to Show/Hide
|
|||||
| BioChemical Class |
Nuclear hormone receptor
|
|||||
| UniProt ID | ||||||
| Sequence |
MGETLGDSPIDPESDSFTDTLSANISQEMTMVDTEMPFWPTNFGISSVDLSVMEDHSHSF
DIKPFTTVDFSSISTPHYEDIPFTRTDPVVADYKYDLKLQEYQSAIKVEPASPPYYSEKT QLYNKPHEEPSNSLMAIECRVCGDKASGFHYGVHACEGCKGFFRRTIRLKLIYDRCDLNC RIHKKSRNKCQYCRFQKCLAVGMSHNAIRFGRMPQAEKEKLLAEISSDIDQLNPESADLR ALAKHLYDSYIKSFPLTKAKARAILTGKTTDKSPFVIYDMNSLMMGEDKIKFKHITPLQE QSKEVAIRIFQGCQFRSVEAVQEITEYAKSIPGFVNLDLNDQVTLLKYGVHEIIYTMLAS LMNKDGVLISEGQGFMTREFLKSLRKPFGDFMEPKFEFAVKFNALELDDSDLAIFIAVII LSGDRPGLLNVKPIEDIQDNLLQALELQLKLNHPESSQLFAKLLQKMTDLRQIVTEHVQL LQVIKKTETDMSLHPLLQEIYKDLY Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | PDB | ||||
| ADReCS ID | BADD_A02556 ; BADD_A04820 ; BADD_A05675 | |||||
| HIT2.0 ID | T58J2Y | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Approved Drug(s) | [+] 5 Approved Drugs | + | ||||
| 1 | Lobeglitazone | Drug Info | Approved | Type-2 diabetes | [2] | |
| 2 | Pioglitazone | Drug Info | Approved | Diabetic complication | [3], [4] | |
| 3 | Rosiglitazone XR | Drug Info | Approved | Type-2 diabetes | [5] | |
| 4 | Thiazolidinedione | Drug Info | Approved | Alzheimer disease | [6] | |
| 5 | Troglitazone | Drug Info | Approved | Diabetic complication | [7], [8] | |
| Clinical Trial Drug(s) | [+] 27 Clinical Trial Drugs | + | ||||
| 1 | Balaglitazone | Drug Info | Phase 3 | Type-1 diabetes | [9] | |
| 2 | CS-038 | Drug Info | Phase 3 | Type-2 diabetes | [10] | |
| 3 | FARGLITAZAR | Drug Info | Phase 3 | Type-1 diabetes | [11], [12] | |
| 4 | Imiglitazar | Drug Info | Phase 3 | Type-2 diabetes | [13], [14] | |
| 5 | Leriglitazone | Drug Info | Phase 3 | Cerebral X-linked adrenoleukodystrophy | [15] | |
| 6 | Ragaglitazar | Drug Info | Phase 3 | Type-1 diabetes | [16], [17] | |
| 7 | Rivoglitazone | Drug Info | Phase 3 | Ocular inflammation | [18] | |
| 8 | Rosiglitazone + metformin | Drug Info | Phase 3 | Diabetic complication | [19] | |
| 9 | Rosiglitazone + simvastatin | Drug Info | Phase 3 | Type-2 diabetes | [20] | |
| 10 | TESAGLITAZAR | Drug Info | Phase 3 | Type-1 diabetes | [21] | |
| 11 | ZYH-1 | Drug Info | Phase 3 | Lipid metabolism disorder | [22] | |
| 12 | MBX-102 | Drug Info | Phase 2/3 | Gout | [23] | |
| 13 | CHS-131 | Drug Info | Phase 2 | Multiple sclerosis | [24] | |
| 14 | FK-614 | Drug Info | Phase 2 | Type-2 diabetes | [25] | |
| 15 | GED-0507-34-Levo | Drug Info | Phase 2 | Inflammatory bowel disease | [26] | |
| 16 | MBX-2044 | Drug Info | Phase 2 | Type-2 diabetes | [27] | |
| 17 | Naveglitazar | Drug Info | Phase 2 | Diabetic complication | [28] | |
| 18 | Netoglitazone | Drug Info | Phase 2 | Non-alcoholic fatty liver disease | [29], [30] | |
| 19 | OMS405 | Drug Info | Phase 2 | Nicotine dependence | [31] | |
| 20 | ONO-5129 | Drug Info | Phase 2 | Diabetic complication | [32] | |
| 21 | T3D-959 | Drug Info | Phase 2 | Alzheimer disease | [33] | |
| 22 | T3D-959 | Drug Info | Phase 1/2 | Alzheimer disease | [34] | |
| 23 | CLX-0921 | Drug Info | Phase 1 | Type-2 diabetes | [35] | |
| 24 | DSP-8658 | Drug Info | Phase 1 | Type-2 diabetes | [36] | |
| 25 | Englitazone sodium | Drug Info | Phase 1 | Type-2 diabetes | [37] | |
| 26 | GW-409544 | Drug Info | Phase 1 | Hyperlipidaemia | [38], [39] | |
| 27 | Oxeglitazar | Drug Info | Phase 1 | Gout | [40] | |
| Discontinued Drug(s) | [+] 18 Discontinued Drugs | + | ||||
| 1 | AVE-0847 | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [41] | |
| 2 | GSK-677954 | Drug Info | Discontinued in Phase 2 | Non-alcoholic fatty liver disease | [30] | |
| 3 | Indeglitazar | Drug Info | Discontinued in Phase 2 | Type-2 diabetes | [42] | |
| 4 | KRP-297 | Drug Info | Discontinued in Phase 2 | Type-2 diabetes | [43] | |
| 5 | Reglixane | Drug Info | Discontinued in Phase 2 | Diabetic complication | [44] | |
| 6 | Sodelglitazar | Drug Info | Discontinued in Phase 2 | Hyperlipidaemia | [45] | |
| 7 | YM-440 | Drug Info | Discontinued in Phase 2 | Type-1 diabetes | [46] | |
| 8 | DS-6930 | Drug Info | Discontinued in Phase 1 | Diabetic complication | [47] | |
| 9 | E-3030 | Drug Info | Discontinued in Phase 1 | Hyperlipidaemia | [48] | |
| 10 | LY-929 | Drug Info | Discontinued in Phase 1 | Lipid metabolism disorder | [49], [50] | |
| 11 | AD-5075 | Drug Info | Terminated | Type-2 diabetes | [53], [54] | |
| 12 | AKP-320 | Drug Info | Terminated | Type-2 diabetes | [55] | |
| 13 | BVT-142 | Drug Info | Terminated | Type-2 diabetes | [56] | |
| 14 | CS-204 | Drug Info | Terminated | Metabolic disorder | [57] | |
| 15 | DARGLITAZONE | Drug Info | Terminated | Diabetic complication | [58] | |
| 16 | Edaglitazone | Drug Info | Terminated | Type-2 diabetes | [59] | |
| 17 | SB-219994 | Drug Info | Terminated | Diabetic complication | [60], [61] | |
| 18 | Sipoglitazar | Drug Info | Terminated | Diabetic complication | [62] | |
| Preclinical Drug(s) | [+] 2 Preclinical Drugs | + | ||||
| 1 | Ciglitazone | Drug Info | Preclinical | Pulmonary fibrosis | [51] | |
| 2 | MC-3002 | Drug Info | Preclinical | Metabolic disorder | [52] | |
| Mode of Action | [+] 4 Modes of Action | + | ||||
| Agonist | [+] 53 Agonist drugs | + | ||||
| 1 | Lobeglitazone | Drug Info | [2] | |||
| 2 | Pioglitazone | Drug Info | [1], [63] | |||
| 3 | Rosiglitazone XR | Drug Info | [64] | |||
| 4 | Thiazolidinedione | Drug Info | [1] | |||
| 5 | Troglitazone | Drug Info | [1], [65], [66] | |||
| 6 | Glitazone | Drug Info | [67] | |||
| 7 | Leriglitazone | Drug Info | [75] | |||
| 8 | Rivoglitazone | Drug Info | [77] | |||
| 9 | Rosiglitazone + metformin | Drug Info | [64] | |||
| 10 | Rosiglitazone + simvastatin | Drug Info | [64] | |||
| 11 | MBX-102 | Drug Info | [27] | |||
| 12 | FK-614 | Drug Info | [25] | |||
| 13 | GED-0507-34-Levo | Drug Info | [79] | |||
| 14 | MBX-2044 | Drug Info | [27] | |||
| 15 | OMS405 | Drug Info | [82] | |||
| 16 | T3D-959 | Drug Info | [84] | |||
| 17 | CLX-0921 | Drug Info | [35] | |||
| 18 | Englitazone sodium | Drug Info | [37], [87] | |||
| 19 | 1-(biphenyl-4-yl-methyl)-1H-imidazole derivative 1 | Drug Info | [88] | |||
| 20 | 3-phenyl acrylic acid derivative 1 | Drug Info | [88] | |||
| 21 | A-substituted phenylpropionic acid derivative 1 | Drug Info | [88] | |||
| 22 | A-substituted phenylpropionic acid derivative 2 | Drug Info | [88] | |||
| 23 | Biaromatic compound 1 | Drug Info | [88] | |||
| 24 | Crystalline anhydrous toluene derivative 1 | Drug Info | [88] | |||
| 25 | Flavonoid derivative 8 | Drug Info | [88] | |||
| 26 | Fused aromatic compound 1 | Drug Info | [88] | |||
| 27 | Fused ring compound 1 | Drug Info | [88] | |||
| 28 | MRL20 | Drug Info | [88] | |||
| 29 | MRL24 | Drug Info | [88] | |||
| 30 | Phenylpropionic acid derivative 1 | Drug Info | [88] | |||
| 31 | Phenylpyridine derivative 3 | Drug Info | [88] | |||
| 32 | PMID25416646-Compound-Figure2-J | Drug Info | [88] | |||
| 33 | PMID25416646-Compound-Figure2-K | Drug Info | [88] | |||
| 34 | PMID25416646-Compound-Figure2-N | Drug Info | [88] | |||
| 35 | PMID25416646-Compound-Figure5-A | Drug Info | [88] | |||
| 36 | PMID25416646-Compound-Figure5-C | Drug Info | [88] | |||
| 37 | PMID25416646-Compound-Figure5-E | Drug Info | [88] | |||
| 38 | PMID25416646-Compound-Figure5-H | Drug Info | [88] | |||
| 39 | Spirolaxine derivative 1 | Drug Info | [88] | |||
| 40 | Sulfonamide derivative 18 | Drug Info | [88] | |||
| 41 | Thiazolidine dione crystalline derivative 1 | Drug Info | [88] | |||
| 42 | GSK-677954 | Drug Info | [30] | |||
| 43 | Indeglitazar | Drug Info | [90] | |||
| 44 | Sodelglitazar | Drug Info | [92] | |||
| 45 | LY-929 | Drug Info | [96] | |||
| 46 | Ciglitazone | Drug Info | [97] | |||
| 47 | BVT-142 | Drug Info | [56], [99] | |||
| 48 | CS-204 | Drug Info | [100] | |||
| 49 | DARGLITAZONE | Drug Info | [101], [87] | |||
| 50 | SB-219994 | Drug Info | [102] | |||
| 51 | 15-deoxy-Delta(12, 14)-prostaglandin J(2) | Drug Info | [65] | |||
| 52 | DB-900 | Drug Info | [104] | |||
| 53 | GW7845 | Drug Info | [106] | |||
| Modulator | [+] 28 Modulator drugs | + | ||||
| 1 | Balaglitazone | Drug Info | [68] | |||
| 2 | CS-038 | Drug Info | [69], [70] | |||
| 3 | FARGLITAZAR | Drug Info | [71], [72] | |||
| 4 | Imiglitazar | Drug Info | [73], [74] | |||
| 5 | MURAGLITAZAR | Drug Info | [76] | |||
| 6 | Ragaglitazar | Drug Info | [17] | |||
| 7 | TESAGLITAZAR | Drug Info | [78] | |||
| 8 | ZYH-1 | Drug Info | [62] | |||
| 9 | CHS-131 | Drug Info | [24] | |||
| 10 | Naveglitazar | Drug Info | [80] | |||
| 11 | Netoglitazone | Drug Info | [81] | |||
| 12 | ONO-5129 | Drug Info | [83] | |||
| 13 | T3D-959 | Drug Info | [85] | |||
| 14 | DSP-8658 | Drug Info | [86] | |||
| 15 | GW-409544 | Drug Info | [39] | |||
| 16 | Oxeglitazar | Drug Info | [40] | |||
| 17 | AVE-0847 | Drug Info | [89] | |||
| 18 | KRP-297 | Drug Info | [91] | |||
| 19 | Reglixane | Drug Info | [83] | |||
| 20 | DS-6930 | Drug Info | [94] | |||
| 21 | E-3030 | Drug Info | [95] | |||
| 22 | MC-3002 | Drug Info | [83] | |||
| 23 | AD-5075 | Drug Info | [54] | |||
| 24 | AKP-320 | Drug Info | [98] | |||
| 25 | Edaglitazone | Drug Info | [59] | |||
| 26 | Sipoglitazar | Drug Info | [62] | |||
| 27 | GW-2331 | Drug Info | [105] | |||
| 28 | ZY H2 | Drug Info | [107] | |||
| Ligand | [+] 1 Ligand drugs | + | ||||
| 1 | Cannabinoid quinone derivative 1 | Drug Info | [88] | |||
| Inhibitor | [+] 8 Inhibitor drugs | + | ||||
| 1 | YM-440 | Drug Info | [93], [87] | |||
| 2 | (2S)-2-(4-chlorophenoxy)-3-phenylpropanoic acid | Drug Info | [103] | |||
| 3 | (2S)-2-(4-ethylphenoxy)-3-phenylpropanoic acid | Drug Info | [103] | |||
| 4 | (2S)-2-(biphenyl-4-yloxy)-3-phenylpropanoic acid | Drug Info | [103] | |||
| 5 | (9Z,11E,13S)-13-hydroxyoctadeca-9,11-dienoic acid | Drug Info | [103] | |||
| 6 | 2-chloro-5-nitro-N-phenylbenzamide | Drug Info | [103] | |||
| 7 | 3-(5-methoxy-1H-indol-3-yl)propanoic acid | Drug Info | [103] | |||
| 8 | 9-hydroxyoctadecadienoic acid | Drug Info | [103] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Indomethacin | Ligand Info | |||||
| Structure Description | Human PPARgamma ligand-binding domain in complex with indomethacin and nitro-233 | PDB:3ADX | ||||
| Method | X-ray diffraction | Resolution | 1.95 Å | Mutation | No | [108] |
| PDB Sequence |
QLNPESADLR
212 ALAKHLYDSY222 IKSFPLTKAK232 ARAILTGKTT242 DKSPFVIYDM252 NSLMMGEDKI 262 KFKHITPLQE272 QSKEVAIRIF282 QGCQFRSVEA292 VQEITEYAKS302 IPGFVNLDLN 312 DQVTLLKYGV322 HEIIYTMLAS332 LMNKDGVLIS342 EGQGFMTREF352 LKSLRKPFGD 362 FMEPKFEFAV372 KFNALELDDS382 DLAIFIAVII392 LSGDRPGLLN402 VKPIEDIQDN 412 LLQALELQLK422 LNHPESSQLF432 AKLLQKMTDL442 RQIVTEHVQL452 LQVIKKTETD 462 MSLHPLLQEI472 YKDL
|
|||||
|
|
ALA278
4.525
ILE281
3.493
PHE282
3.093
CYS285
3.632
GLN286
3.984
SER289
3.015
HIS323
2.880
ILE326
3.351
TYR327
3.109
LEU330
3.923
LEU353
3.662
|
|||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Ligand Name: Glutathione | Ligand Info | |||||
| Structure Description | Human peroxisome proliferator-activated receptor gamma ligand binding domain complexed with glutathion conjugated 15-deoxy-delta12,14-prostaglandin J2 | PDB:2ZK2 | ||||
| Method | X-ray diffraction | Resolution | 2.26 Å | Mutation | No | [109] |
| PDB Sequence |
QLNPESADLR
212 ALAKHLYDSY222 IKSFPLTKAK232 ARAILTGKTT242 DKSPFVIYDM252 NSLMMGEDKI 262 KFKHITPLQE272 QSKEVAIRIF282 QGCQFRSVEA292 VQEITEYAKS302 IPGFVNLDLN 312 DQVTLLKYGV322 HEIIYTMLAS332 LMNKDGVLIS342 EGQGFMTREF352 LKSLRKPFGD 362 FMEPKFEFAV372 KFNALELDDS382 DLAIFIAVII392 LSGDRPGLLN402 VKPIEDIQDN 412 LLQALELQLK422 LNHPESSQLF432 AKLLQKMTDL442 RQIVTEHVQL452 LQVIKKTETD 462 MSLHPLLQEI472 YKDL
|
|||||
|
|
LEU228
4.365
PHE264
3.738
ILE267
3.833
THR268
4.497
ARG280
3.628
ILE281
3.078
PHE282
4.922
GLN283
4.767
GLY284
2.935
CYS285
3.473
ARG288
2.887
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
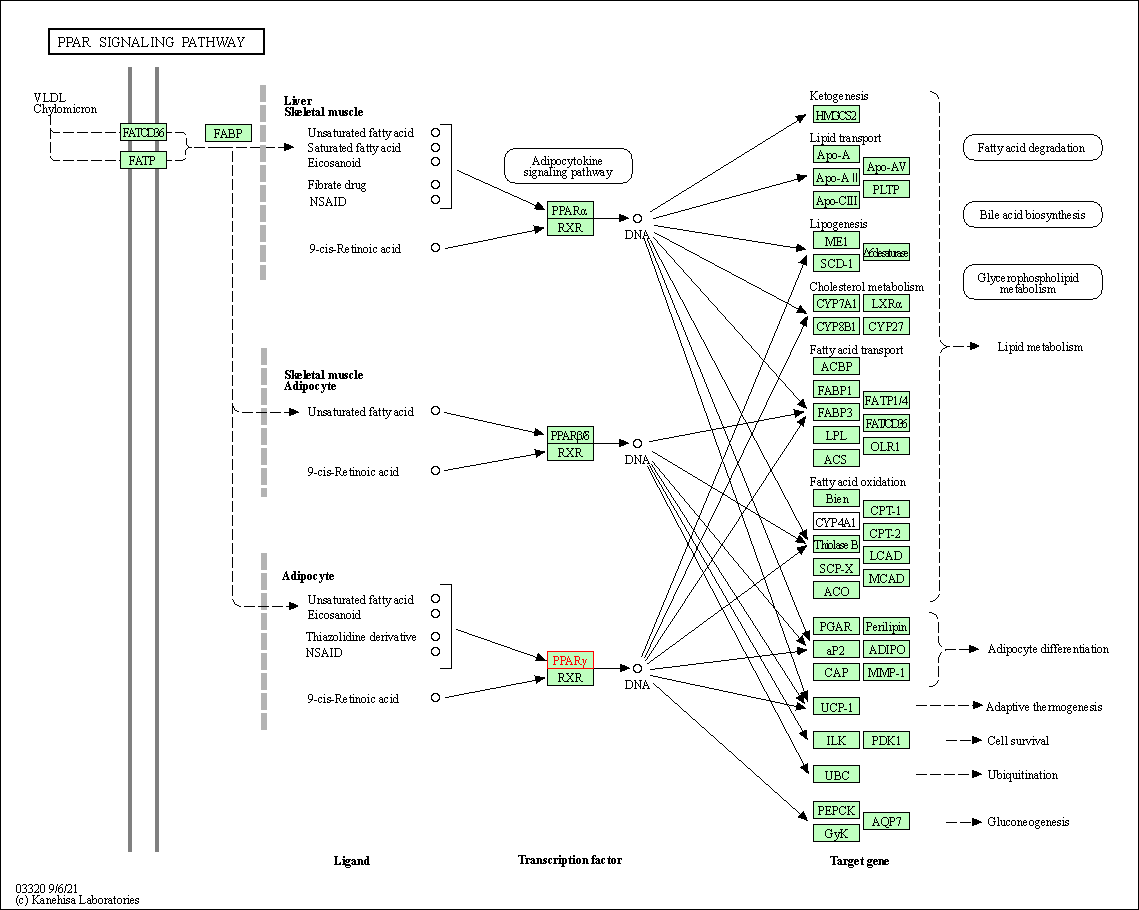
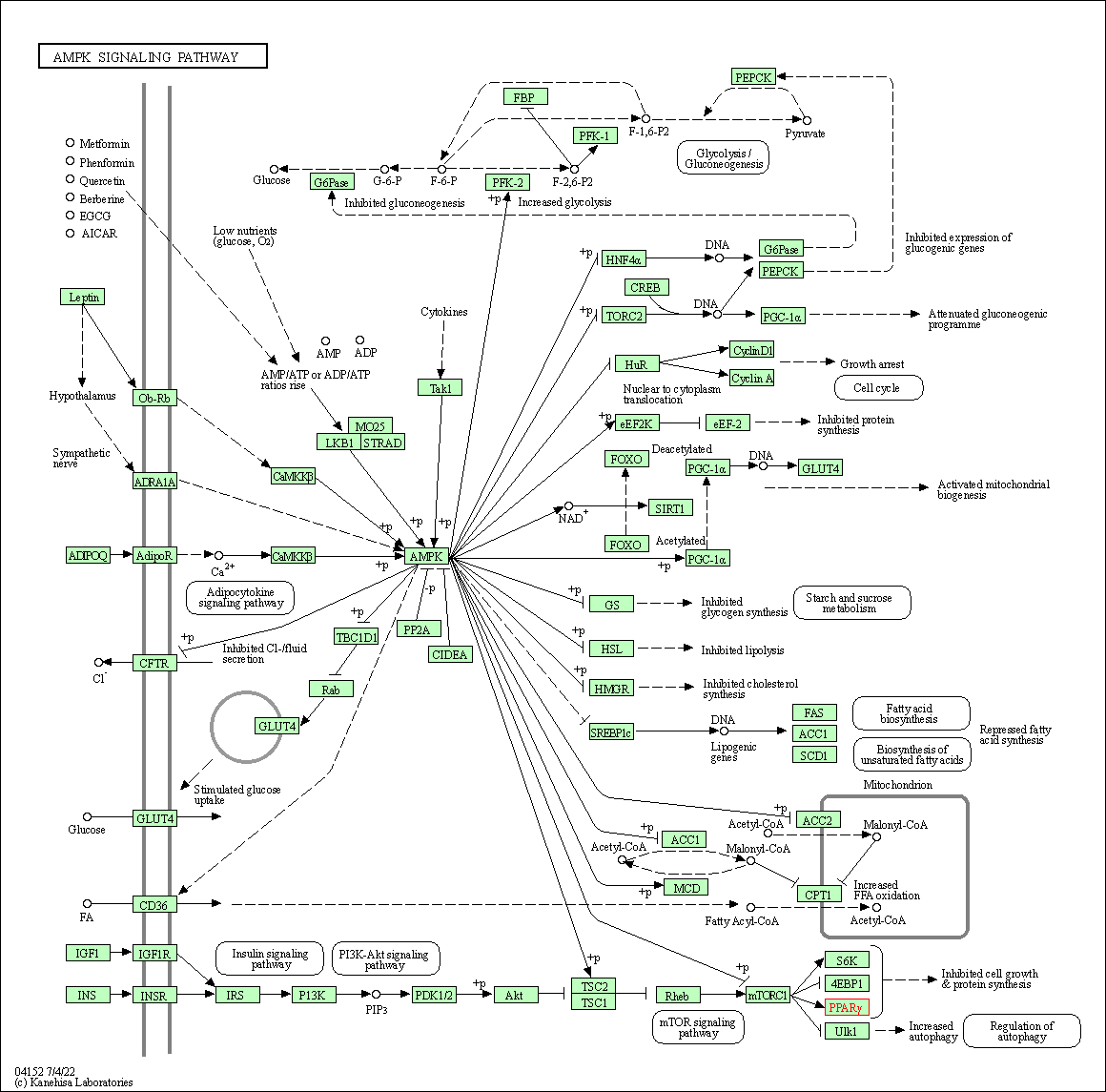
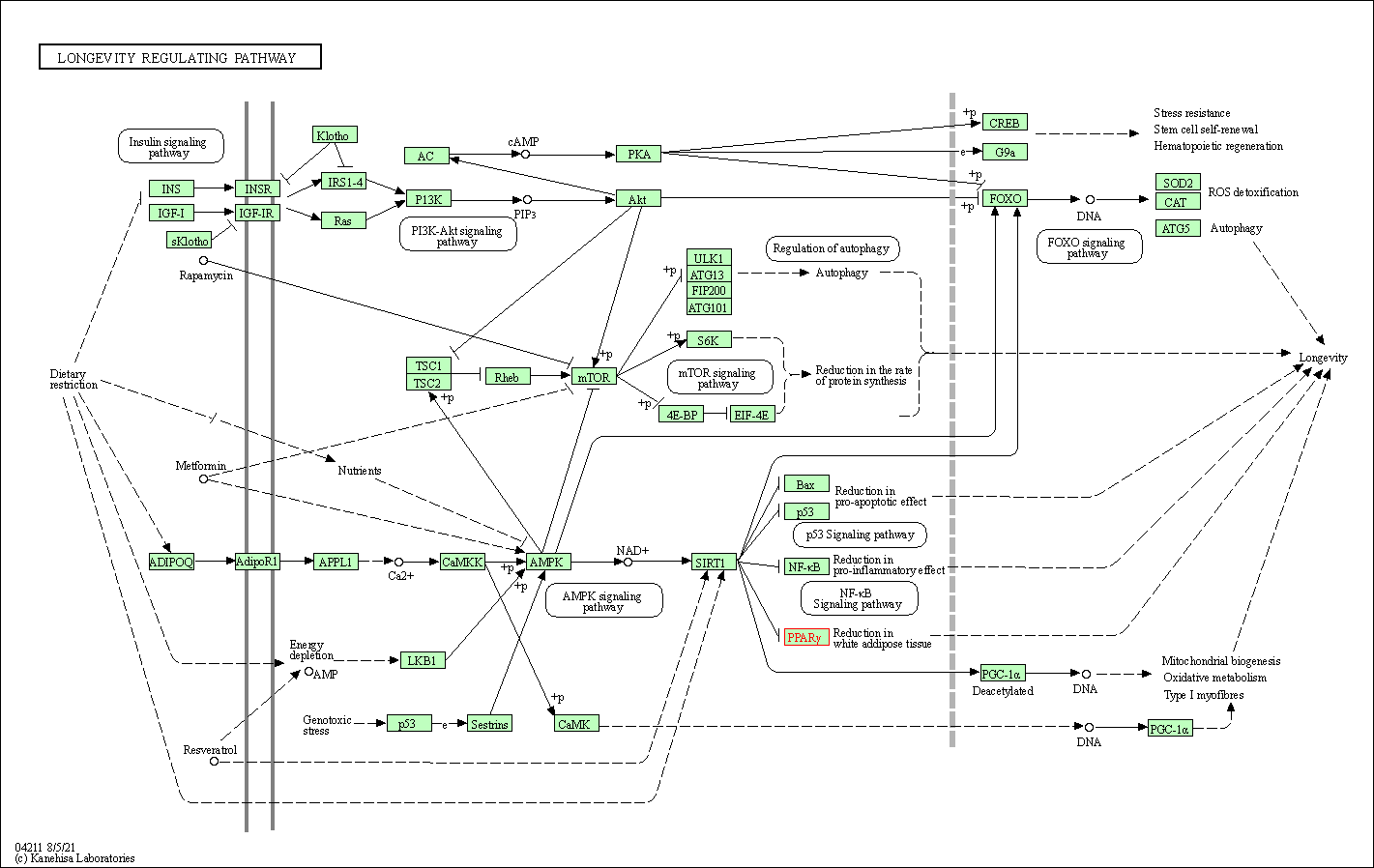
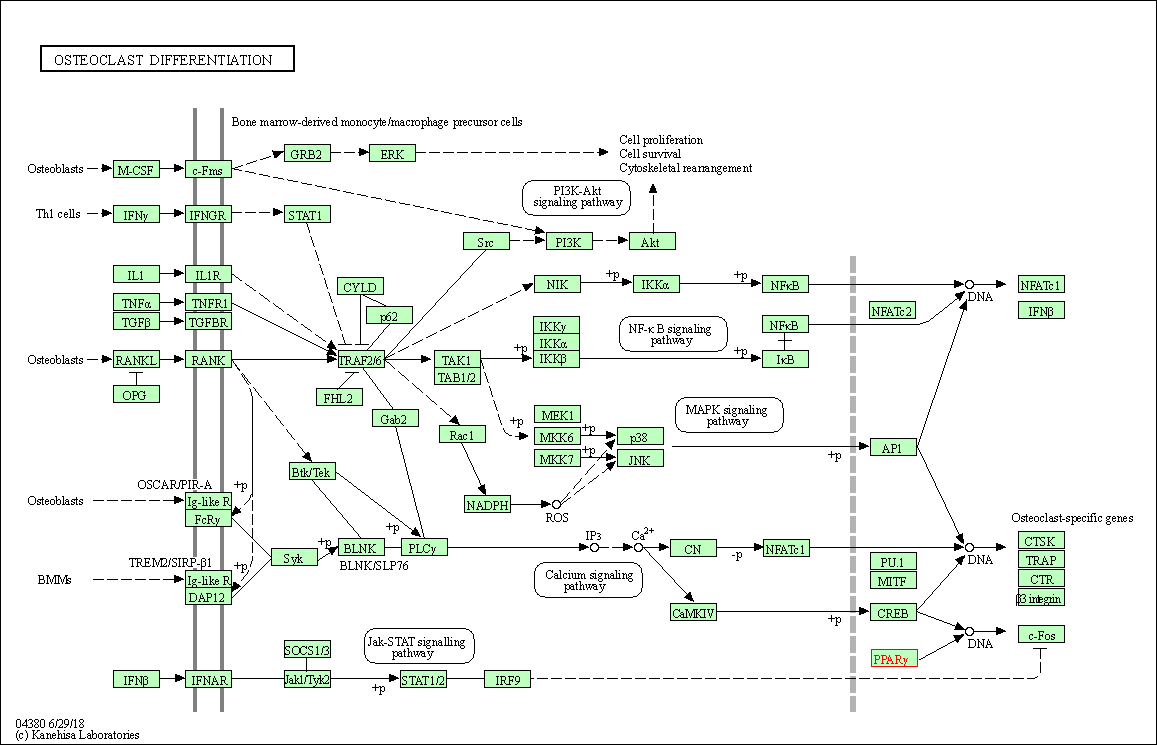

| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| PPAR signaling pathway | hsa03320 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| AMPK signaling pathway | hsa04152 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Longevity regulating pathway | hsa04211 | Affiliated Target |

|
| Class: Organismal Systems => Aging | Pathway Hierarchy | ||
| Osteoclast differentiation | hsa04380 | Affiliated Target |

|
| Class: Organismal Systems => Development and regeneration | Pathway Hierarchy | ||
| Thermogenesis | hsa04714 | Affiliated Target |

|
| Class: Organismal Systems => Environmental adaptation | Pathway Hierarchy | ||
| Degree | 52 | Degree centrality | 5.59E-03 | Betweenness centrality | 4.21E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.58E-01 | Radiality | 1.45E+01 | Clustering coefficient | 9.95E-02 |
| Neighborhood connectivity | 3.50E+01 | Topological coefficient | 4.04E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Functional PPAR-gamma receptor is a novel therapeutic target for ACTH-secreting pituitary adenomas. Nat Med. 2002 Nov;8(11):1281-7. | |||||
| REF 2 | Tolerability and pharmacokinetics of lobeglitazone, a novel peroxisome proliferator-activated receptor-gamma agonist, after a single oral administration in healthy female subjects. Clin Drug Investig. 2014 Jul;34(7):467-74. | |||||
| REF 3 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2694). | |||||
| REF 4 | Emerging drug candidates of dipeptidyl peptidase IV (DPP IV) inhibitor class for the treatment of Type 2 Diabetes. Curr Drug Targets. 2009 Jan;10(1):71-87. | |||||
| REF 5 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||||
| REF 6 | Thiazolidinediones: a comparative review of approved uses. Diabetes Technol Ther. 2000 Autumn;2(3):429-40. | |||||
| REF 7 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2693). | |||||
| REF 8 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||||
| REF 9 | ClinicalTrials.gov (NCT00515632) Efficacy and Safety of Treatment With Balaglitazone in Type 2 Diabetes Patients on Stable Insulin Therapy. U.S. National Institutes of Health. | |||||
| REF 10 | ClinicalTrials.gov (NCT02173457) Study of Chiglitazar Compare With Sitagliptin in Type 2 Diabetes Patients. U.S. National Institutes of Health. | |||||
| REF 11 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2672). | |||||
| REF 12 | Clinical pipeline report, company report or official report of MacroGenics. | |||||
| REF 13 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2675). | |||||
| REF 14 | Medicinal Chemistry and Actions of Dual and Pan PPAR Modulators. Open Med Chem J. 2011;5(Suppl 2):93-8. | |||||
| REF 15 | ClinicalTrials.gov (NCT05819866) A Clinical Study to Assess the Efficacy and Safety of Leriglitazone in Adult Male Subjects With Cerebral Adrenoleukodystrophy. U.S.National Institutes of Health. | |||||
| REF 16 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2664). | |||||
| REF 17 | Ragaglitazar: the pharmacokinetics, pharmacodynamics, and tolerability of a novel dual PPAR alpha and gamma agonist in healthy subjects and patients with type 2 diabetes. J Clin Pharmacol. 2003 Nov;43(11):1244-56. | |||||
| REF 18 | ClinicalTrials.gov (NCT00571519) Randomized, Double-blind, Active-controlled, Study of Rivoglitazone in Type 2 Diabetes Mellitus. U.S. National Institutes of Health. | |||||
| REF 19 | ClinicalTrials.gov (NCT00499707) Efficacy and Safety Study of Rosiglitazone/Metformin Therapy vs Rosiglitazone and Metformin in Type 2 Diabetes Subjects. U.S. National Institutes of Health. | |||||
| REF 20 | Clinical pipeline report, company report or official report of GlaxoSmithKline. | |||||
| REF 21 | ClinicalTrials.gov (NCT00229710) GALLEX 9: Safety and Tolerability of Oral Tesaglitazar When Added to Insulin Therapy in Patients With Type 2 Diabetes. U.S. National Institutes of Health. | |||||
| REF 22 | A multicenter, prospective, randomized, double-blind study to evaluate the safety and efficacy of Saroglitazar 2 and 4 g compared with placebo in type 2 diabetes mellitus patients having hypertriglyceridemia not controlled with atorvastatin therapy (PRESS VI). Diabetes Technol Ther. 2014 Feb;16(2):63-71. | |||||
| REF 23 | ClinicalTrials.gov (NCT00353587) Safety and Efficacy Study of Metaglidasen in Type 2 Diabetes in Patients Suboptimally Controlled on Insulin. U.S. National Institutes of Health. | |||||
| REF 24 | Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA) | |||||
| REF 25 | FK-614, a selective peroxisome proliferator-activated receptor gamma agonist, improves peripheral glucose utilization while decreasing hepatic insulin extraction in alloxan-induced diabetic dogs. Metabolism. 2005 Sep;54(9):1250-8. | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800032997) | |||||
| REF 27 | MBX-102/JNJ39659100, a novel peroxisome proliferator-activated receptor-ligand with weak transactivation activity retains antidiabetic properties in the absence of weight gain and edema. Mol Endocrinol. 2009 Jul;23(7):975-88. | |||||
| REF 28 | ClinicalTrials.gov (NCT00065312) An Evaluation of an Oral Antidiabetic Agent for the Treatment of Type 2 Diabetes. U.S. National Institutes of Health. | |||||
| REF 29 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2707). | |||||
| REF 30 | Emerging drugs for non-alcoholic fatty liver disease. Expert Opin Emerg Drugs. 2008 Mar;13(1):145-58. | |||||
| REF 31 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800035962) | |||||
| REF 32 | ClinicalTrials.gov (NCT00335712) Pilot Study of ONO-5129 in Patients With Type 2 Diabetes Mellitus. U.S. National Institutes of Health. | |||||
| REF 33 | ClinicalTrials.gov (NCT04251182) A Randomized, Double-Blind, Placebo-Controlled Multi-Center Study to Evaluate the Safety and Efficacy of Three Dose Strengths of T3D-959 in Subjects With Mild-to-Moderate Alzheimer's Disease. U.S.National Institutes of Health. | |||||
| REF 34 | ClinicalTrials.gov (NCT02560753) Feasibility Study in Subjects With Mild to Moderate Alzheimer's Disease. | |||||
| REF 35 | A novel peroxisome proliferator-activated gamma (PPAR gamma) agonist, CLX-0921, has potent antihyperglycemic activity with low adipogenic potential. Metabolism. 2003 Aug;52(8):1012-8. | |||||
| REF 36 | ClinicalTrials.gov (NCT01042106) Safety, Pharmacokinetics and Pharmacodynamics of DSP-8658 in Patients With Type 2 Diabetes Mellitus and Healthy Adults. U.S. National Institutes of Health. | |||||
| REF 37 | PPARgamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long-term efficacy. Int J Obes Relat Metab Disord. 2003 Feb;27(2):147-61. | |||||
| REF 38 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3440). | |||||
| REF 39 | Design novel dual agonists for treating type-2 diabetes by targeting peroxisome proliferator-activated receptors with core hopping approach. PLoS One. 2012;7(6):e38546. | |||||
| REF 40 | Therapeutic potential of aleglitazar, a new dual PPAR-alpha/gamma agonist: implications for cardiovascular disease in patients with diabetes mellitus. Am J Cardiovasc Drugs. 2010;10(4):209-16. | |||||
| REF 41 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800020608) | |||||
| REF 42 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022483) | |||||
| REF 43 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010490) | |||||
| REF 44 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800008953) | |||||
| REF 45 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800018093) | |||||
| REF 46 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800010082) | |||||
| REF 47 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800036558) | |||||
| REF 48 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021476) | |||||
| REF 49 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2657). | |||||
| REF 50 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800014823) | |||||
| REF 51 | Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov. 2020 Jan;19(1):57-75. | |||||
| REF 52 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800021065) | |||||
| REF 53 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2701). | |||||
| REF 54 | Thiazolidinediones produce a conformational change in peroxisomal proliferator-activated receptor-gamma: binding and activation correlate with antidiabetic actions in db/db mice. Endocrinology. 1996 Oct;137(10):4189-95. | |||||
| REF 55 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022081) | |||||
| REF 56 | A new class of peroxisome proliferator-activated receptor agonists with a novel binding epitope shows antidiabetic effects. J Biol Chem. 2004 Sep 24;279(39):41124-30. | |||||
| REF 57 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800026847) | |||||
| REF 58 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800003002) | |||||
| REF 59 | A specific peroxisome proliferator-activated receptor-gamma (PPAR-gamma) ligand, pioglitazone, ameliorates gastric mucosal damage induced by ischemia and reperfusion in rats. Redox Rep. 2002;7(5):343-6. | |||||
| REF 60 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2698). | |||||
| REF 61 | Identification of high-affinity binding sites for the insulin sensitizer rosiglitazone (BRL-49653) in rodent and human adipocytes using a radioiodinated ligand for peroxisomal proliferator-activated receptor gamma. J Pharmacol Exp Ther. 1998 Feb;284(2):751-9. | |||||
| REF 62 | Pharmacokinetics, safety, and tolerability of saroglitazar (ZYH1), a predominantly PPARalpha agonist with moderate PPARgamma agonist activity in healthy human subjects. Clin Drug Investig. 2013 Nov;33(11):809-16. | |||||
| REF 63 | Obesity: pathophysiology and clinical management. Curr Med Chem. 2009;16(4):506-21. | |||||
| REF 64 | Clinical pipeline report, company report or official report of GlaxoSmithKline (2009). | |||||
| REF 65 | Expression of peroxisome proliferator-activated receptors (PPARs) in human urinary bladder carcinoma and growth inhibition by its agonists. Int J Cancer. 2003 May 1;104(5):597-602. | |||||
| REF 66 | Knockouts model the 100 best-selling drugs--will they model the next 100 Nat Rev Drug Discov. 2003 Jan;2(1):38-51. | |||||
| REF 67 | Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000 Jun 2;275(22):16638-42. | |||||
| REF 68 | Balaglitazone: a second generation peroxisome proliferator-activated receptor (PPAR) gamma () agonist.Mini Rev Med Chem.2012 Feb;12(2):87-97. | |||||
| REF 69 | Determination of chiglitazar, a dual alpha/gamma peroxisome proliferator-activated receptor (PPAR) agonist, in human plasma by liquid chromatograph... Pharmazie. 2007 Nov;62(11):825-9. | |||||
| REF 70 | The PPARalpha/gamma dual agonist chiglitazar improves insulin resistance and dyslipidemia in MSG obese rats.Br J Pharmacol.2006 Jul;148(5):610-8. | |||||
| REF 71 | Rosiglitazone (BRL49653), a PPARgamma-selective agonist, causes peroxisome proliferator-like liver effects in obese mice. J Lipid Res. 1999 Jul;40(7):1177-84. | |||||
| REF 72 | Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces pulmonary inflammatory response in a rat model of endotoxemia. Inflamm Res. 2005 Nov;54(11):464-70. | |||||
| REF 73 | A novel oxyiminoalkanoic acid derivative, TAK-559, activates human peroxisome proliferator-activated receptor subtypes. Eur J Pharmacol. 2004 Jul 8;495(1):17-26. | |||||
| REF 74 | A potent activator of PPARalpha and gamma reduces the vascular cell recruitment and inhibits the intimal thickning in hypercholesterolemic rabbits. Atherosclerosis. 2005 Jan;178(1):1-7. | |||||
| REF 75 | Clinical pipeline report, company report or official report of Minoryx Therapeutics | |||||
| REF 76 | Muraglitazar, a dual (alpha/gamma) PPAR activator: a randomized, double-blind, placebo-controlled, 24-week monotherapy trial in adult patients with... Clin Ther. 2005 Aug;27(8):1181-95. | |||||
| REF 77 | A randomized-controlled trial to investigate the effects of rivoglitazone, a novel PPAR gamma agonist on glucose-lipid control in type 2 diabetes. Diabetes Obes Metab. 2011 Sep;13(9):806-13. | |||||
| REF 78 | Tesaglitazar, a dual PPAR-/ agonist, hamster carcinogenicity, investigative animal and clinical studies.Toxicol Pathol.2012;40(1):18-32. | |||||
| REF 79 | PPAR-gamma in ulcerative colitis: a novel target for intervention. Curr Drug Targets. 2013 Nov;14(12):1501-7. | |||||
| REF 80 | The disposition and metabolism of naveglitazar, a peroxisome proliferator-activated receptor alpha-gamma dual, gamma-dominant agonist in mice, rats... Drug Metab Dispos. 2007 Jan;35(1):51-61. | |||||
| REF 81 | Netoglitazone is a PPAR-gamma ligand with selective effects on bone and fat. Bone. 2006 Jan;38(1):74-84. | |||||
| REF 82 | Clinical pipeline report, company report or official report of Avarx. | |||||
| REF 83 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 84 | T3D-959: A Multi-Faceted Disease Remedial Drug Candidate for the Treatment of Alzheimer's Disease. J Alzheimers Dis. 2016;51(1):123-38. | |||||
| REF 85 | Phrma report (2013 Alzheimers disease) | |||||
| REF 86 | Effects of DSP-8658, a Novel Selective Peroxisome Proliferator-activated Receptors a/gamma Modulator, on Adipogenesis and Glucose Metabolism in Diabetic Obese Mice. Exp Clin Endocrinol Diabetes. 2015Sep;123(8):492-9. | |||||
| REF 87 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | |||||
| REF 88 | PPAR ligands and their therapeutic applications: a patent review (2008 - 2014).Expert Opin Ther Pat. 2015 Feb;25(2):175-91. | |||||
| REF 89 | DOI: 10.1038/scibx.2012.669 | |||||
| REF 90 | Scaffold-based discovery of indeglitazar, a PPAR pan-active anti-diabetic agent. Proc Natl Acad Sci U S A. 2009 Jan 6;106(1):262-7. | |||||
| REF 91 | KRP-297, MCC-555. Nihon Rinsho. 2001 Nov;59(11):2200-6. | |||||
| REF 92 | Docking and molecular dynamics simulations of peroxisome proliferator activated receptors interacting with pan agonist sodelglitazar. Protein Pept Lett. 2011 Oct;18(10):1021-7. | |||||
| REF 93 | The novel hypoglycemic agent YM440 improves hepatic insulin resistance in obese Zucker fatty rats. J Pharmacol Sci. 2006 Aug;101(4):311-7. | |||||
| REF 94 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800036558) | |||||
| REF 95 | Antidiabetic and hypolipidemic effects of a novel dual peroxisome proliferator-activated receptor (PPAR) alpha/gamma agonist, E3030, in db/db mice and beagle dogs.J Pharmacol Sci.2008 Sep;108(1):40-8. | |||||
| REF 96 | CN patent application no. 1882326, Ppar agonists for the treatment of hcv infection. | |||||
| REF 97 | New drugs in development for the treatment of endometriosis. Expert Opin Investig Drugs. 2008 Aug;17(8):1187-202. | |||||
| REF 98 | Chronic glucose-lowering effects of rosiglitazone and bis(ethylmaltolato) oxovanadium(IV) in ZDF rats. Canadian Journal of Physiology and Pharmacology. 12/2003; 81(11):1049-55. | |||||
| REF 99 | US patent application no. 7,816,328, Substituted fused heterocyclic c-glycosides. | |||||
| REF 100 | Peroxisome Proliferators-Activated Receptor (PPAR) Modulators and Metabolic Disorders. PPAR Res. 2008; 2008: 679137. | |||||
| REF 101 | The PPAR-gamma agonist, darglitazone, restores acute inflammatory responses to cerebral hypoxia-ischemia in the diabetic ob/ob mouse. J Cereb Blood Flow Metab. 2010 February; 30(2): 352-360. | |||||
| REF 102 | PPAR-gamma agonists as therapy for diseases involving airway neutrophilia. Eur Respir J. 2004 Jul;24(1):18-23. | |||||
| REF 103 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 104 | CN patent application no. 102459215, 3-(4-aminophenyl)-2-furancarboxylic acid derivative and pharmaceutically acceptable salt thereof. | |||||
| REF 105 | PPAR-alpha and -gamma but not -delta agonists inhibit airway inflammation in a murine model of asthma: in vitro evidence for an NF-kappaB-independe... Br J Pharmacol. 2003 May;139(1):163-71. | |||||
| REF 106 | A new ligand for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 1999 Nov 15;59(22):5671-3. | |||||
| REF 107 | The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954. | |||||
| REF 108 | The nuclear receptor PPAR individually responds to serotonin- and fatty acid-metabolites. EMBO J. 2010 Oct 6;29(19):3395-407. | |||||
| REF 109 | Structural insight into PPARgamma activation through covalent modification with endogenous fatty acids. J Mol Biol. 2009 Jan 9;385(1):188-99. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

