Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T82393
(Former ID: TTDR00847)
|
|||||
| Target Name |
TNF alpha converting enzyme (ADAM17)
|
|||||
| Synonyms |
TNFalpha converting enzyme; TNF-alpha-converting enzyme; TNF-alpha converting enzyme; TNF-alpha convertase; TACE; Snake venom-like protease; Disintegrin and metalloproteinase domain-containing protein 17; CSVP; CD156b antigen; CD156b; ADAM 17; A disintegrin and metalloproteinase domain 17
Click to Show/Hide
|
|||||
| Gene Name |
ADAM17
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Rheumatoid arthritis [ICD-11: FA20] | |||||
| 2 | Breast cancer [ICD-11: 2C60-2C6Y] | |||||
| Function |
Responsible for the proteolytical release of soluble JAM3 from endothelial cells surface. Responsible for the proteolytic release of several other cell-surface proteins, including p75 TNF-receptor, interleukin 1 receptor type II, p55 TNF-receptor, transforming growth factor-alpha, L-selectin, growth hormone receptor, MUC1 and the amyloid precursor protein. Acts as an activator of Notch pathway by mediating cleavage of Notch, generating the membrane-associated intermediate fragment called Notch extracellular truncation (NEXT). Plays a role in the proteolytic processing of ACE2. Plays a role in hemostasis through shedding of GP1BA, the platelet glycoprotein Ib alpha chain. Mediates the proteolytic cleavage of LAG3, leading to release the secreted form of LAG3. Cleaves the membrane-bound precursor of TNF-alpha to its mature soluble form.
Click to Show/Hide
|
|||||
| BioChemical Class |
Peptidase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 3.4.24.86
|
|||||
| Sequence |
MRQSLLFLTSVVPFVLAPRPPDDPGFGPHQRLEKLDSLLSDYDILSLSNIQQHSVRKRDL
QTSTHVETLLTFSALKRHFKLYLTSSTERFSQNFKVVVVDGKNESEYTVKWQDFFTGHVV GEPDSRVLAHIRDDDVIIRINTDGAEYNIEPLWRFVNDTKDKRMLVYKSEDIKNVSRLQS PKVCGYLKVDNEELLPKGLVDREPPEELVHRVKRRADPDPMKNTCKLLVVADHRFYRYMG RGEESTTTNYLIELIDRVDDIYRNTSWDNAGFKGYGIQIEQIRILKSPQEVKPGEKHYNM AKSYPNEEKDAWDVKMLLEQFSFDIAEEASKVCLAHLFTYQDFDMGTLGLAYVGSPRANS HGGVCPKAYYSPVGKKNIYLNSGLTSTKNYGKTILTKEADLVTTHELGHNFGAEHDPDGL AECAPNEDQGGKYVMYPIAVSGDHENNKMFSNCSKQSIYKTIESKAQECFQERSNKVCGN SRVDEGEECDPGIMYLNNDTCCNSDCTLKEGVQCSDRNSPCCKNCQFETAQKKCQEAINA TCKGVSYCTGNSSECPPPGNAEDDTVCLDLGKCKDGKCIPFCEREQQLESCACNETDNSC KVCCRDLSGRCVPYVDAEQKNLFLRKGKPCTVGFCDMNGKCEKRVQDVIERFWDFIDQLS INTFGKFLADNIVGSVLVFSLIFWIPFSILVHCVDKKLDKQYESLSLFHPSNVEMLSSMD SASVRIIKPFPAPQTPGRLQPAPVIPSAPAAPKLDHQRMDTIQEDPSTDSHMDEDGFEKD PFPNSSTAAKSFEDLTDHPVTRSEKAASFKLQRQNRVDSKETEC Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T91MWA | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 2 Clinical Trial Drugs | + | ||||
| 1 | Apratastat | Drug Info | Phase 2 | Rheumatoid arthritis | [2], [3] | |
| 2 | Aderbasib | Drug Info | Phase 1/2 | Breast cancer | [4] | |
| Preclinical Drug(s) | [+] 1 Preclinical Drugs | + | ||||
| 1 | GW-3333 | Drug Info | Preclinical | Chronic obstructive pulmonary disease | [5] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | DPC-333 | Drug Info | Terminated | Rheumatoid arthritis | [5], [6] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Modulator | [+] 2 Modulator drugs | + | ||||
| 1 | Apratastat | Drug Info | [1], [7], [8] | |||
| 2 | Aderbasib | Drug Info | [9] | |||
| Inhibitor | [+] 15 Inhibitor drugs | + | ||||
| 1 | GW-3333 | Drug Info | [5] | |||
| 2 | DPC-333 | Drug Info | [5] | |||
| 3 | 2-(4-bromophenylsulfonamido)-N-hydroxyacetamide | Drug Info | [10] | |||
| 4 | 2-(biphenyl-4-ylsulfonamido)-N-hydroxyacetamide | Drug Info | [10] | |||
| 5 | Batimistat | Drug Info | [11] | |||
| 6 | CH4474 | Drug Info | [12] | |||
| 7 | IK-862 | Drug Info | [13] | |||
| 8 | IM-491 | Drug Info | [14] | |||
| 9 | N-hydroxy-2-(4-methoxyphenylsulfonamido)acetamide | Drug Info | [10] | |||
| 10 | N-hydroxy-3-(2-oxo-2H-chromen-3-yl)propanamide | Drug Info | [15] | |||
| 11 | N-hydroxy-3-(6-methoxy-2-oxo-2H-chromen-3-yl) | Drug Info | [15] | |||
| 12 | PKF-241-466 | Drug Info | [16] | |||
| 13 | PKF-242-484 | Drug Info | [16] | |||
| 14 | SL422 | Drug Info | [17] | |||
| 15 | SR-973 | Drug Info | [18] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: IK-862 | Ligand Info | |||||
| Structure Description | Crystal structure of TACE in complex with IK682 | PDB:2FV5 | ||||
| Method | X-ray diffraction | Resolution | 2.10 Å | Mutation | Yes | [19] |
| PDB Sequence |
ADPDPMKNTC
225 KLLVVADHRF235 YRYMGRGEES245 TTTNYLIELI255 DRVDDIYRNT265 AWDNAGFKGY 275 GIQIEQIRIL285 KSPQEVKPGE295 KHYNMAKSYP305 NEEKDAWDVK315 MLLEQFSFDI 325 AEEASKVCLA335 HLFTYQDFDM345 GTLGLAYGGS355 PRANSHGGVC365 PKAYYSPVGK 375 KNIYLNSGLT385 STKNYGKTIL395 TKEADLVTTH405 ELGHNFGAEH415 DPDGLAECAP 425 NEDQGGKYVM435 YPIAVSGDHE445 NNKMFSQCSK455 QSIYKTIESK465 AQECFQERSN 475 A
|
|||||
|
|
GLY346
3.437
THR347
3.206
LEU348
2.632
GLY349
2.886
LEU350
3.793
ALA351
4.575
GLU398
3.505
LEU401
3.257
VAL402
4.100
HIS405
3.040
GLU406
2.527
HIS409
3.007
|
|||||
| Ligand Name: N-{[4-(but-2-yn-1-yloxy)phenyl]sulfonyl}-5-methyl-D-tryptophan | Ligand Info | |||||
| Structure Description | Crystal Structure of TACE with Tryptophan Sulfonamide Derivative Inhibitor | PDB:3G42 | ||||
| Method | X-ray diffraction | Resolution | 2.10 Å | Mutation | Yes | [20] |
| PDB Sequence |
MKNTCKLLVV
230 ADHRFYRYMG240 RGEESTTTNY250 LIELIDRVDD260 IYRNTAWDNA270 GFKGYGIQIE 280 QIRILKSPQE290 VKPGEKHYNM300 AKSYPNEEKD310 AWDVKMLLEQ320 FSFDIAEEAS 330 KVCLAHLFTY340 QDFDMGTLGL350 AYVGSPRANS360 HGGVCPKAYY370 SPVGKKNIYL 380 NSGLTSTKNY390 GKTILTKEAD400 LVTTHELGHN410 FGAEHDPDGL420 AECAPNEDQG 430 GKYVMYPIAV440 SGDHENNKMF450 SQCSKQSIYK460 TIESKAQECF470 QERS |
|||||
|
|
GLY346
4.046
THR347
3.226
LEU348
2.777
GLY349
3.213
LEU350
3.910
ALA351
3.975
VAL353
3.692
GLU398
3.989
LEU401
3.701
VAL402
3.913
|
|||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
There is no similarity protein (E value < 0.005) for this target
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
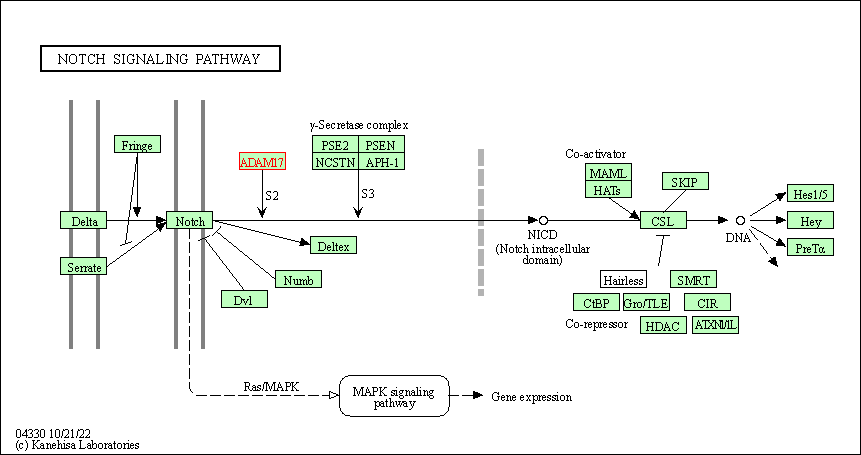
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Notch signaling pathway | hsa04330 | Affiliated Target |

|
| Class: Environmental Information Processing => Signal transduction | Pathway Hierarchy | ||
| Degree | 14 | Degree centrality | 1.50E-03 | Betweenness centrality | 4.57E-04 |
|---|---|---|---|---|---|
| Closeness centrality | 2.26E-01 | Radiality | 1.40E+01 | Clustering coefficient | 1.10E-01 |
| Neighborhood connectivity | 2.11E+01 | Topological coefficient | 9.73E-02 | Eccentricity | 11 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| Target QSAR Model | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Acetylenic TACE inhibitors. Part 3: Thiomorpholine sulfonamide hydroxamates. Bioorg Med Chem Lett. 2006 Mar 15;16(6):1605-9. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6482). | |||||
| REF 3 | ClinicalTrials.gov (NCT00095342) Study Evaluating TMI-005 in Active Rheumatoid Arthritis. U.S. National Institutes of Health. | |||||
| REF 4 | ClinicalTrials.gov (NCT02141451) INCB7839 With Rituximab After Autologous Hematopoietic Cell Transplantation for Diffuse Large B Cell Non-Hodgkin Lymphoma. U.S. National Institutes of Health. | |||||
| REF 5 | Emerging drugs for the treatment of chronic obstructive pulmonary disease. Expert Opin Emerg Drugs. 2006 May;11(2):275-91. | |||||
| REF 6 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6509). | |||||
| REF 7 | Drug insight: tumor necrosis factor-converting enzyme as a pharmaceutical target for rheumatoid arthritis. Nat Clin Pract Rheumatol. 2008 Jun;4(6):300-9. | |||||
| REF 8 | Drug evaluation: apratastat, a novel TACE/MMP inhibitor for rheumatoid arthritis. Curr Opin Investig Drugs. 2006 Nov;7(11):1014-9. | |||||
| REF 9 | Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41. | |||||
| REF 10 | Potent arylsulfonamide inhibitors of tumor necrosis factor-alpha converting enzyme able to reduce activated leukocyte cell adhesion molecule sheddi... J Med Chem. 2010 Mar 25;53(6):2622-35. | |||||
| REF 11 | The secretases that cleave angiotensin converting enzyme and the amyloid precursor protein are distinct from tumour necrosis factor-alpha convertase. FEBS Lett. 1998 Jul 10;431(1):63-5. | |||||
| REF 12 | Tumour necrosis factor-alpha converting enzyme (TACE) activity in human colonic epithelial cells. Clin Exp Immunol. 2004 Jan;135(1):146-53. | |||||
| REF 13 | Specific targeting of metzincin family members with small-molecule inhibitors: progress toward a multifarious challenge. Bioorg Med Chem. 2008 Oct 1;16(19):8781-94. | |||||
| REF 14 | Discovery of beta-benzamido hydroxamic acids as potent, selective, and orally bioavailable TACE inhibitors. Bioorg Med Chem Lett. 2008 Jan 1;18(1):241-6. | |||||
| REF 15 | Chromen-based TNF-alpha converting enzyme (TACE) inhibitors: design, synthesis, and biological evaluation. Bioorg Med Chem. 2008 Jan 1;16(1):530-5. | |||||
| REF 16 | Current perspective of TACE inhibitors: a review. Bioorg Med Chem. 2009 Jan 15;17(2):444-59. | |||||
| REF 17 | Design, synthesis, and structure-activity relationships of macrocyclic hydroxamic acids that inhibit tumor necrosis factor alpha release in vitro and in vivo. J Med Chem. 2001 Aug 2;44(16):2636-60. | |||||
| REF 18 | Synthesis and evaluation of succinoyl-caprolactam gamma-secretase inhibitors. Bioorg Med Chem Lett. 2006 May 1;16(9):2357-63. | |||||
| REF 19 | IK682, a tight binding inhibitor of TACE. Arch Biochem Biophys. 2006 Jul 1;451(1):43-50. | |||||
| REF 20 | Synthesis and activity of tryptophan sulfonamide derivatives as novel non-hydroxamate TNF-alpha converting enzyme (TACE) inhibitors. Bioorg Med Chem. 2009 Jun 1;17(11):3857-65. | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

