Target Information
| Target General Information | Top | |||||
|---|---|---|---|---|---|---|
| Target ID |
T87675
(Former ID: TTDC00006)
|
|||||
| Target Name |
Aurora kinase A (AURKA)
|
|||||
| Synonyms |
hARK1; Serine/threonine-protein kinase aurora-A; Serine/threonine-protein kinase 6; Serine/threonine-protein kinase 15; Serine/threonine kinase 15; STK6; STK15; IAK1; Breast tumor-amplified kinase; BTAK; Aurora/IPL1-related kinase 1; Aurora-related kinase 1; Aurora-A; Aurora 2; AYK1; AURA; ARK1; ARK-1; AIRK1; AIK
Click to Show/Hide
|
|||||
| Gene Name |
AURKA
|
|||||
| Target Type |
Clinical trial target
|
[1] | ||||
| Disease | [+] 2 Target-related Diseases | + | ||||
| 1 | Acute diabete complication [ICD-11: 5A2Y] | |||||
| 2 | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||||
| Function |
Associates with the centrosome and the spindle microtubules during mitosis and plays a critical role in various mitotic events including the establishment of mitotic spindle, centrosome duplication, centrosome separation as well as maturation, chromosomal alignment, spindle assembly checkpoint, and cytokinesis. Required for normal spindle positioning during mitosis and for the localization of NUMA1 and DCTN1 to the cell cortex during metaphase. Required for initial activation of CDK1 at centrosomes. Phosphorylates numerous target proteins, including ARHGEF2, BORA, BRCA1, CDC25B, DLGP5, HDAC6, KIF2A, LATS2, NDEL1, PARD3, PPP1R2, PLK1, RASSF1, TACC3, p53/TP53 and TPX2. Regulates KIF2A tubulin depolymerase activity. Required for normal axon formation. Plays a role in microtubule remodeling during neurite extension. Important for microtubule formation and/or stabilization. Also acts as a key regulatory component of the p53/TP53 pathway, and particularly the checkpoint-response pathways critical for oncogenic transformation of cells, by phosphorylating and stabilizing p53/TP53. Phosphorylates its own inhibitors, the protein phosphatase type 1 (PP1) isoforms, to inhibit their activity. Necessary for proper cilia disassembly prior to mitosis. Mitotic serine/threonine kinase that contributes to the regulation of cell cycle progression.
Click to Show/Hide
|
|||||
| BioChemical Class |
Kinase
|
|||||
| UniProt ID | ||||||
| EC Number |
EC 2.7.11.1
|
|||||
| Sequence |
MDRSKENCISGPVKATAPVGGPKRVLVTQQFPCQNPLPVNSGQAQRVLCPSNSSQRVPLQ
AQKLVSSHKPVQNQKQKQLQATSVPHPVSRPLNNTQKSKQPLPSAPENNPEEELASKQKN EESKKRQWALEDFEIGRPLGKGKFGNVYLAREKQSKFILALKVLFKAQLEKAGVEHQLRR EVEIQSHLRHPNILRLYGYFHDATRVYLILEYAPLGTVYRELQKLSKFDEQRTATYITEL ANALSYCHSKRVIHRDIKPENLLLGSAGELKIADFGWSVHAPSSRRTTLCGTLDYLPPEM IEGRMHDEKVDLWSLGVLCYEFLVGKPPFEANTYQETYKRISRVEFTFPDFVTEGARDLI SRLLKHNPSQRPMLREVLEHPWITANSSKPSNCQNKESASKQS Click to Show/Hide
|
|||||
| 3D Structure | Click to Show 3D Structure of This Target | AlphaFold | ||||
| HIT2.0 ID | T80DCQ | |||||
| Drugs and Modes of Action | Top | |||||
|---|---|---|---|---|---|---|
| Clinical Trial Drug(s) | [+] 17 Clinical Trial Drugs | + | ||||
| 1 | AT9283 | Drug Info | Phase 3 | Solid tumour/cancer | [2], [3] | |
| 2 | MLN8237 | Drug Info | Phase 3 | Solid tumour/cancer | [4], [5] | |
| 3 | Rosiglitazone + metformin | Drug Info | Phase 3 | Diabetic complication | [6] | |
| 4 | ABT-348 | Drug Info | Phase 2 | Haematological malignancy | [7] | |
| 5 | ENMD-2076 | Drug Info | Phase 2 | Acute myeloid leukaemia | [8], [9] | |
| 6 | PHA-739358 | Drug Info | Phase 2 | Prostate cancer | [10], [11] | |
| 7 | VX-680 | Drug Info | Phase 2 | Solid tumour/cancer | [12], [13] | |
| 8 | LY3295668 | Drug Info | Phase 1/2 | Solid tumour/cancer | [14] | |
| 9 | AMG 900 | Drug Info | Phase 1 | Solid tumour/cancer | [15], [16] | |
| 10 | CYC116 | Drug Info | Phase 1 | Solid tumour/cancer | [17] | |
| 11 | HPP-607 | Drug Info | Phase 1 | Solid tumour/cancer | [18] | |
| 12 | MK-5108 | Drug Info | Phase 1 | Solid tumour/cancer | [19] | |
| 13 | MLN8054 | Drug Info | Phase 1 | Solid tumour/cancer | [20], [21] | |
| 14 | R763 | Drug Info | Phase 1 | Haematological malignancy | [22] | |
| 15 | SNS-314 | Drug Info | Phase 1 | Solid tumour/cancer | [23] | |
| 16 | TAS-119 | Drug Info | Phase 1 | Solid tumour/cancer | [24] | |
| 17 | VIC-1911 | Drug Info | Phase 1 | Solid tumour/cancer | [25] | |
| Discontinued Drug(s) | [+] 1 Discontinued Drugs | + | ||||
| 1 | PF-03814735 | Drug Info | Discontinued in Phase 1 | Advanced solid tumour | [26] | |
| Mode of Action | [+] 2 Modes of Action | + | ||||
| Modulator | [+] 3 Modulator drugs | + | ||||
| 1 | AT9283 | Drug Info | [3] | |||
| 2 | ABT-348 | Drug Info | [29] | |||
| 3 | AMG 900 | Drug Info | [16] | |||
| Inhibitor | [+] 24 Inhibitor drugs | + | ||||
| 1 | MLN8237 | Drug Info | [1] | |||
| 2 | Rosiglitazone + metformin | Drug Info | [27], [28] | |||
| 3 | ENMD-2076 | Drug Info | [30] | |||
| 4 | PHA-739358 | Drug Info | [30] | |||
| 5 | VX-680 | Drug Info | [27], [31] | |||
| 6 | LY3295668 | Drug Info | [32] | |||
| 7 | CYC116 | Drug Info | [33] | |||
| 8 | HPP-607 | Drug Info | [34] | |||
| 9 | MK-5108 | Drug Info | [35], [36] | |||
| 10 | MLN8054 | Drug Info | [27], [37], [38] | |||
| 11 | R763 | Drug Info | [39] | |||
| 12 | SNS-314 | Drug Info | [40], [41], [42], [43] | |||
| 13 | TAS-119 | Drug Info | [44] | |||
| 14 | VIC-1911 | Drug Info | [25] | |||
| 15 | PF-03814735 | Drug Info | [30] | |||
| 16 | 2-(1H-pyrazol-3-yl)-1H-benzimidazole | Drug Info | [45] | |||
| 17 | 4-(1H-pyrazol-4-yl)-1H-pyrrolo[2,3-b]pyridine | Drug Info | [46] | |||
| 18 | 6-bromoindirubin-3-oxime | Drug Info | [47] | |||
| 19 | 7-fluoroindirubin-3-oxime | Drug Info | [47] | |||
| 20 | Indirubin-3'-monoxime | Drug Info | [47] | |||
| 21 | Indirubin-3-acetoxime | Drug Info | [47] | |||
| 22 | Indirubin-3-methoxime | Drug Info | [47] | |||
| 23 | Phosphonothreonine | Drug Info | [48] | |||
| 24 | ZM-447439 | Drug Info | [49] | |||
| Cell-based Target Expression Variations | Top | |||||
|---|---|---|---|---|---|---|
| Cell-based Target Expression Variations | ||||||
| Drug Binding Sites of Target | Top | |||||
|---|---|---|---|---|---|---|
| Ligand Name: Ammonia | Ligand Info | |||||
| Structure Description | Crystal structure of Aurora-A bound to a hydrocarbon-stapled proteomimetic of TPX2 | PDB:5LXM | ||||
| Method | X-ray diffraction | Resolution | 2.08 Å | Mutation | Yes | [50] |
| PDB Sequence |
RQWALEDFEI
135 GRPLGKGKFG145 NVYLAREKQS155 KFILALKVLF165 KAQLEKAGVE175 HQLRREVEIQ 185 SHLRHPNILR195 LYGYFHDATR205 VYLILEYAPL215 GTVYRELQKL225 SKFDEQRTAT 235 YITELANALS245 YCHSKRVIHR255 DIKPENLLLG265 SAGELKIADF275 GWSVHAPSSR 285 RTLAGTLDYL296 PPEMIEGRMH306 DEKVDLWSLG316 VLCYEFLVGK326 PPFEANTYQE 336 TYKRISRVEF346 TFPDFVTEGA356 RDLISRLLKH366 NPSQRPMLRE376 VLEHPWITAN 386 SSKPSN
|
|||||
|
|
||||||
| Ligand Name: Adenosine | Ligand Info | |||||
| Structure Description | Crystal structures of human kinase Aurora A | PDB:4O0S | ||||
| Method | X-ray diffraction | Resolution | 2.50 Å | Mutation | No | [51] |
| PDB Sequence |
RQWALEDFEI
135 GRPLGKGKFG145 NVYLAREKQS155 KFILALKVLF165 KAQLEKAGVE175 HQLRREVEIQ 185 SHLRHPNILR195 LYGYFHDATR205 VYLILEYAPL215 GTVYRELQKL225 SKFDEQRTAT 235 YITELANALS245 YCHSKRVIHR255 DIKPENLLLG265 SAGELKIADF275 GWSVHAPSSR 285 RTLCGTLDYL296 PPEMIEGRMH306 DEKVDLWSLG316 VLCYEFLVGK326 PPFEANTYQE 336 TYKRISRVEF346 TFPDFVTEGA356 RDLISRLLKH366 NPSQRPMLRE376 VLEHPWITAN 386 SSKPS
|
|||||
|
|
||||||
| Click to View More Binding Site Information of This Target and Ligand Pair | ||||||
| Click to View More Binding Site Information of This Target with Different Ligands | ||||||
| Different Human System Profiles of Target | Top |
|---|---|
|
Human Similarity Proteins
of target is determined by comparing the sequence similarity of all human proteins with the target based on BLAST. The similarity proteins for a target are defined as the proteins with E-value < 0.005 and outside the protein families of the target.
A target that has fewer human similarity proteins outside its family is commonly regarded to possess a greater capacity to avoid undesired interactions and thus increase the possibility of finding successful drugs
(Brief Bioinform, 21: 649-662, 2020).
Human Tissue Distribution
of target is determined from a proteomics study that quantified more than 12,000 genes across 32 normal human tissues. Tissue Specificity (TS) score was used to define the enrichment of target across tissues.
The distribution of targets among different tissues or organs need to be taken into consideration when assessing the target druggability, as it is generally accepted that the wider the target distribution, the greater the concern over potential adverse effects
(Nat Rev Drug Discov, 20: 64-81, 2021).
Human Pathway Affiliation
of target is determined by the life-essential pathways provided on KEGG database. The target-affiliated pathways were defined based on the following two criteria (a) the pathways of the studied target should be life-essential for both healthy individuals and patients, and (b) the studied target should occupy an upstream position in the pathways and therefore had the ability to regulate biological function.
Targets involved in a fewer pathways have greater likelihood to be successfully developed, while those associated with more human pathways increase the chance of undesirable interferences with other human processes
(Pharmacol Rev, 58: 259-279, 2006).
Biological Network Descriptors
of target is determined based on a human protein-protein interactions (PPI) network consisting of 9,309 proteins and 52,713 PPIs, which were with a high confidence score of ≥ 0.95 collected from STRING database.
The network properties of targets based on protein-protein interactions (PPIs) have been widely adopted for the assessment of target’s druggability. Proteins with high node degree tend to have a high impact on network function through multiple interactions, while proteins with high betweenness centrality are regarded to be central for communication in interaction networks and regulate the flow of signaling information
(Front Pharmacol, 9, 1245, 2018;
Curr Opin Struct Biol. 44:134-142, 2017).
Human Similarity Proteins
Human Tissue Distribution
Human Pathway Affiliation
Biological Network Descriptors
|
|
|
Note:
If a protein has TS (tissue specficity) scores at least in one tissue >= 2.5, this protein is called tissue-enriched (including tissue-enriched-but-not-specific and tissue-specific). In the plots, the vertical lines are at thresholds 2.5 and 4.
|
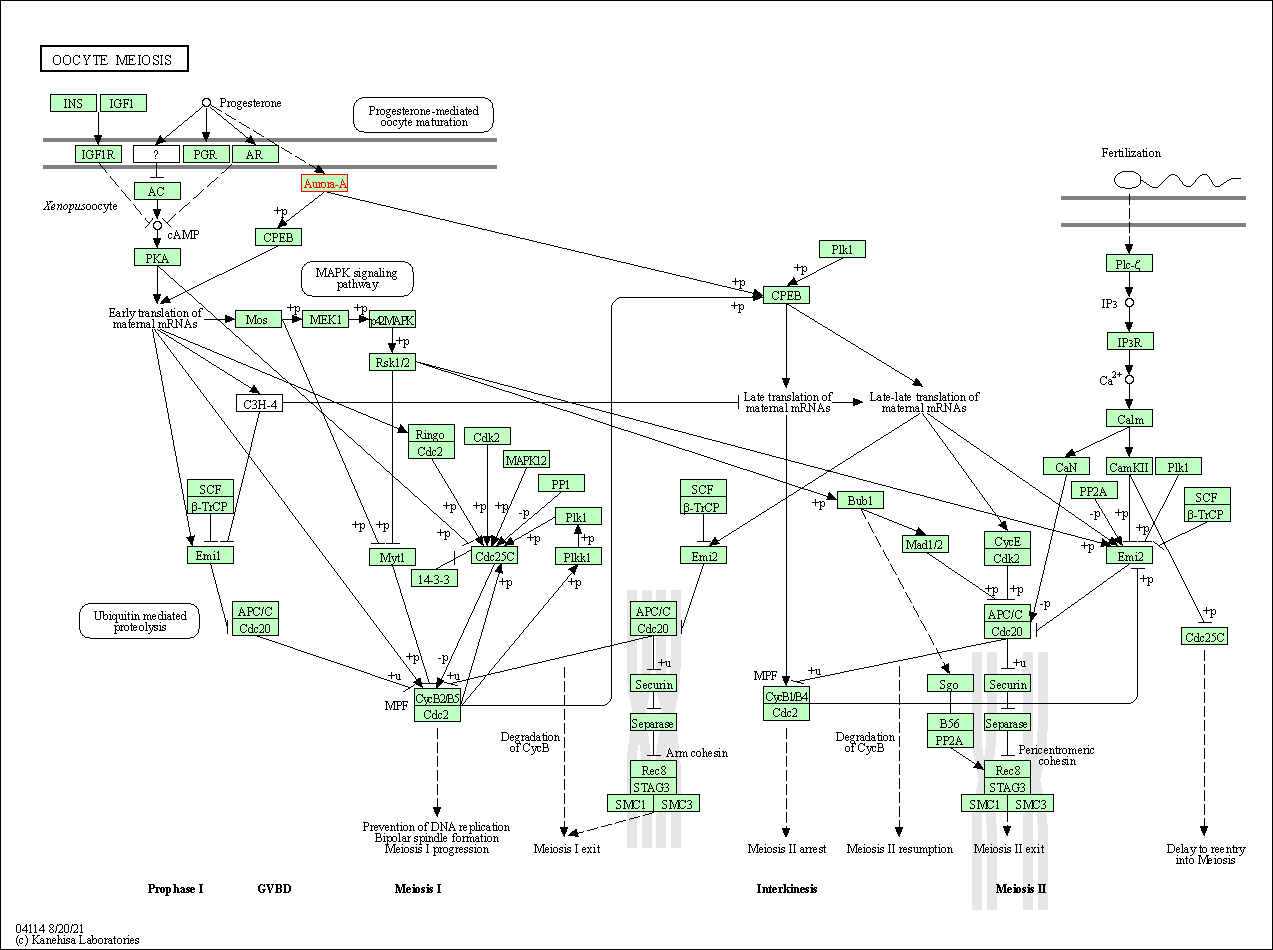
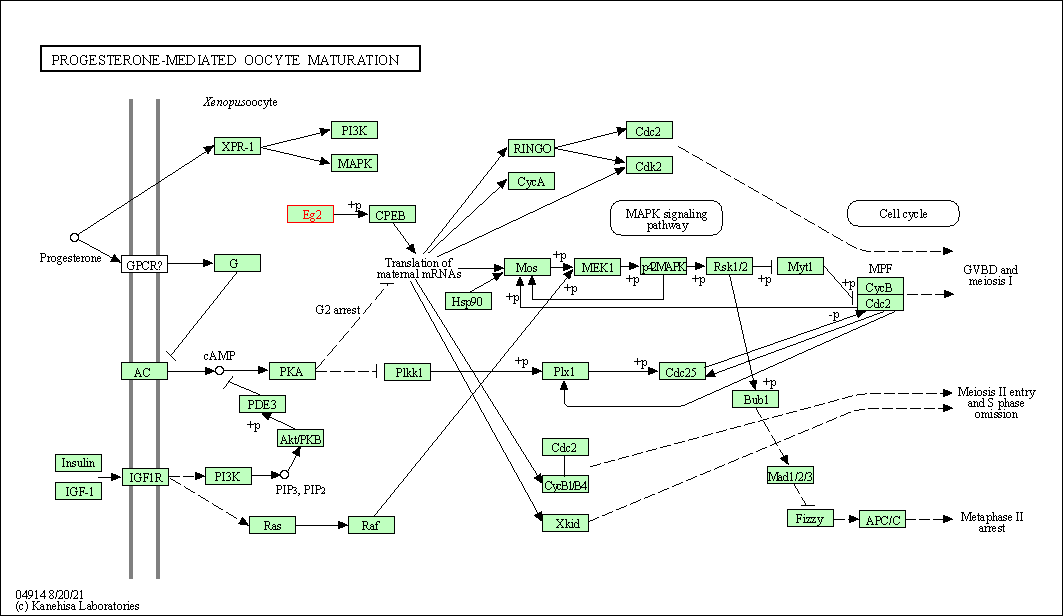
| KEGG Pathway | Pathway ID | Affiliated Target | Pathway Map |
|---|---|---|---|
| Oocyte meiosis | hsa04114 | Affiliated Target |

|
| Class: Cellular Processes => Cell growth and death | Pathway Hierarchy | ||
| Progesterone-mediated oocyte maturation | hsa04914 | Affiliated Target |

|
| Class: Organismal Systems => Endocrine system | Pathway Hierarchy | ||
| Degree | 47 | Degree centrality | 5.05E-03 | Betweenness centrality | 3.27E-03 |
|---|---|---|---|---|---|
| Closeness centrality | 2.45E-01 | Radiality | 1.43E+01 | Clustering coefficient | 3.43E-01 |
| Neighborhood connectivity | 4.21E+01 | Topological coefficient | 6.46E-02 | Eccentricity | 12 |
| Download | Click to Download the Full PPI Network of This Target | ||||
| Chemical Structure based Activity Landscape of Target | Top |
|---|---|
| Drug Property Profile of Target | Top | |
|---|---|---|
| (1) Molecular Weight (mw) based Drug Clustering | (2) Octanol/Water Partition Coefficient (xlogp) based Drug Clustering | |
|
|
||
| (3) Hydrogen Bond Donor Count (hbonddonor) based Drug Clustering | (4) Hydrogen Bond Acceptor Count (hbondacc) based Drug Clustering | |
|
|
||
| (5) Rotatable Bond Count (rotbonds) based Drug Clustering | (6) Topological Polar Surface Area (polararea) based Drug Clustering | |
|
|
||
| "RO5" indicates the cutoff set by lipinski's rule of five; "D123AB" colored in GREEN denotes the no violation of any cutoff in lipinski's rule of five; "D123AB" colored in PURPLE refers to the violation of only one cutoff in lipinski's rule of five; "D123AB" colored in BLACK represents the violation of more than one cutoffs in lipinski's rule of five | ||
| Co-Targets | Top | |||||
|---|---|---|---|---|---|---|
| Co-Targets | ||||||
| Target Poor or Non Binders | Top | |||||
|---|---|---|---|---|---|---|
| Target Poor or Non Binders | ||||||
| Target Regulators | Top | |||||
|---|---|---|---|---|---|---|
| Target-regulating microRNAs | ||||||
| Target-interacting Proteins | ||||||
| Target Profiles in Patients | Top | |||||
|---|---|---|---|---|---|---|
| Target Expression Profile (TEP) |
||||||
| Target Affiliated Biological Pathways | Top | |||||
|---|---|---|---|---|---|---|
| KEGG Pathway | [+] 1 KEGG Pathways | + | ||||
| 1 | Oocyte meiosis | |||||
| PID Pathway | [+] 5 PID Pathways | + | ||||
| 1 | Aurora B signaling | |||||
| 2 | Signaling by Aurora kinases | |||||
| 3 | Integrin-linked kinase signaling | |||||
| 4 | PLK1 signaling events | |||||
| 5 | Aurora A signaling | |||||
| Reactome | [+] 2 Reactome Pathways | + | ||||
| 1 | APC/C:Cdh1 mediated degradation of Cdc20 and other APC/C:Cdh1 targeted proteins in late mitosis/early G1 | |||||
| 2 | Regulation of PLK1 Activity at G2/M Transition | |||||
| WikiPathways | [+] 5 WikiPathways | + | ||||
| 1 | EGF/EGFR Signaling Pathway | |||||
| 2 | JAK/STAT | |||||
| 3 | Gastric Cancer Network 1 | |||||
| 4 | Integrated Breast Cancer Pathway | |||||
| 5 | APC/C-mediated degradation of cell cycle proteins | |||||
| Target-Related Models and Studies | Top | |||||
|---|---|---|---|---|---|---|
| Target Validation | ||||||
| References | Top | |||||
|---|---|---|---|---|---|---|
| REF 1 | Effect of Aurora A kinase inhibitor MLN8237 combined with rituximab on antitumor activity in preclinical B-cell non-Hodgkin's lymphoma models. Journal of Clinical Oncology, 2009:8553. | |||||
| REF 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7949). | |||||
| REF 3 | A phase I trial of AT9283 (a selective inhibitor of aurora kinases) in children and adolescents with solid tumors: a Cancer Research UK study. Clin Cancer Res. 2015 Jan 15;21(2):267-73. | |||||
| REF 4 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7790). | |||||
| REF 5 | ClinicalTrials.gov (NCT01482962) Alisertib (MLN8237) or Investigator's Choice in Patients With Relapsed/Refractory Peripheral T-Cell Lymphoma. U.S. National Institutes of Health. | |||||
| REF 6 | ClinicalTrials.gov (NCT00499707) Efficacy and Safety Study of Rosiglitazone/Metformin Therapy vs Rosiglitazone and Metformin in Type 2 Diabetes Subjects. U.S. National Institutes of Health. | |||||
| REF 7 | ClinicalTrials.gov (NCT02478320) Phase II Study of Ilorasertib (ABT348) in Patients With CDKN2A Deficient Solid Tumors. | |||||
| REF 8 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7885). | |||||
| REF 9 | ENMD-2076 is an orally active kinase inhibitor with antiangiogenic and antiproliferative mechanisms of action. Mol Cancer Ther. 2011 Jan;10(1):126-37. | |||||
| REF 10 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7937). | |||||
| REF 11 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025382) | |||||
| REF 12 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5718). | |||||
| REF 13 | VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004 Mar;10(3):262-7. | |||||
| REF 14 | ClinicalTrials.gov (NCT03092934) A Study of AK-01 (LY3295668) in Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 15 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8060). | |||||
| REF 16 | Preclinical evaluation of AMG 900, a novel potent and highly selective pan-aurora kinase inhibitor with activity in taxane-resistant tumor cell lines. Cancer Res. 2010 Dec 1;70(23):9846-54. | |||||
| REF 17 | ClinicalTrials.gov (NCT00560716) A Phase I Pharmacologic Study of CYC116, an Oral Aurora Kinase Inhibitor, in Patients With Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 18 | ClinicalTrials.gov (NCT00939172) TTP607 in Refractory Solid Malignancies. U.S. National Institutes of Health. | |||||
| REF 19 | ClinicalTrials.gov (NCT00543387) Treatment of Participants With Advanced and/or Refractory Solid Tumors (MK-5108-001 AM4). U.S. National Institutes of Health. | |||||
| REF 20 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5696). | |||||
| REF 21 | ClinicalTrials.gov (NCT00652158) A Phase 1 Trial of Extended MLN8054 Dosing in Patients With Advanced Malignancies. U.S. National Institutes of Health. | |||||
| REF 22 | Clinical pipeline report, company report or official report of Rigel. | |||||
| REF 23 | ClinicalTrials.gov (NCT00519662) Safety and Tolerability Study of SNS-314 for Advanced Solid Tumors. U.S. National Institutes of Health. | |||||
| REF 24 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800040598) | |||||
| REF 25 | Clinical pipeline report, company report or official report of VITRAC Therapeutics. | |||||
| REF 26 | Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800025628) | |||||
| REF 27 | A comparison of physicochemical property profiles of marketed oral drugs and orally bioavailable anti-cancer protein kinase inhibitors in clinical development. Curr Top Med Chem. 2007;7(14):1408-22. | |||||
| REF 28 | Dose-finding study of the multitargeted tyrosine kinase inhibitor SU6668 in patients with advanced malignancies. Clin Cancer Res. 2005 Sep 1;11(17):6240-6. | |||||
| REF 29 | Preclinical characterization of ABT-348, a kinase inhibitor targeting the aurora, vascular endothelial growth factor receptor/platelet-derived growth factor receptor, and Src kinase families. J Pharmacol Exp Ther. 2012 Dec;343(3):617-27. | |||||
| REF 30 | Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov. 2009 Jul;8(7):547-66. | |||||
| REF 31 | Essential roles of mTOR/Akt pathway in Aurora-A cell transformation. Int J Biol Sci. 2009 Jun 19;5(5):444-50. | |||||
| REF 32 | Aurora A-Selective Inhibitor LY3295668 Leads to Dominant Mitotic Arrest, Apoptosis in Cancer Cells, and Shows Potent Preclinical Antitumor Efficacy. Mol Cancer Ther. 2019 Dec;18(12):2207-2219. | |||||
| REF 33 | Clinical pipeline report, company report or official report of Cyclacel. | |||||
| REF 34 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Target id: 1936). | |||||
| REF 35 | A novel c-Met inhibitor, MK8033, synergizes with carboplatin plus paclitaxel to inhibit ovarian cancer cell growth. Oncol Rep. 2013 May;29(5):2011-8. | |||||
| REF 36 | Anticancer activity of the Aurora A kinase inhibitor MK-5108 in non-small-cell lung cancer (NSCLC) in vitro as monotherapy and in combination with chemotherapies. J Cancer Res Clin Oncol. 2014 Jul;140(7):1137-49. | |||||
| REF 37 | Clinical pipeline report, company report or official report of Takeda (2009). | |||||
| REF 38 | MLN8054, a small-molecule inhibitor of Aurora A, causes spindle pole and chromosome congression defects leading to aneuploidy. Mol Cell Biol. 2007 Jun;27(12):4513-25. | |||||
| REF 39 | Preclinical characterization of Aurora kinase inhibitor R763/AS703569 identified through an image-based phenotypic screen. J Cancer Res Clin Oncol. 2010 Jan;136(1):99-113. | |||||
| REF 40 | SNS-314, a pan-Aurora kinase inhibitor, shows potent anti-tumor activity and dosing flexibility in vivo. Cancer Chemother Pharmacol. 2010 Mar;65(4):707-17. | |||||
| REF 41 | The Aurora kinase inhibitor SNS-314 shows broad therapeutic potential with chemotherapeutics and synergy with microtubule-targeted agents in a colon carcinoma model. Mol Cancer Ther. 2009 Apr;8(4):930-9. | |||||
| REF 42 | Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2009 Jan-Feb;31(1):47-57. | |||||
| REF 43 | Water-soluble prodrugs of an Aurora kinase inhibitor. Bioorg Med Chem Lett. 2009 Mar 1;19(5):1409-12. | |||||
| REF 44 | TAS-119, a selective Aurora A inhibitor, enhanced the antitumor efficacy of taxanes in multiple human tumor cell lines including paclitaxel-resistant cells. Molecular Cancer Therapeutics. 01/2014; 12(11_Supplement):A268-A268. | |||||
| REF 45 | The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235-42. | |||||
| REF 46 | Discovery of a new series of Aurora inhibitors through truncation of GSK1070916. Bioorg Med Chem Lett. 2010 Apr 15;20(8):2552-5. | |||||
| REF 47 | An integrated computational approach to the phenomenon of potent and selective inhibition of aurora kinases B and C by a series of 7-substituted in... J Med Chem. 2007 Aug 23;50(17):4027-37. | |||||
| REF 48 | How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6. | |||||
| REF 49 | Accurate prediction of the relative potencies of members of a series of kinase inhibitors using molecular docking and MM-GBSA scoring. J Med Chem. 2006 Aug 10;49(16):4805-8. | |||||
| REF 50 | A TPX2 Proteomimetic Has Enhanced Affinity for Aurora-A Due to Hydrocarbon Stapling of a Helix. ACS Chem Biol. 2016 Dec 16;11(12):3383-3390. | |||||
| REF 51 | Functional Role of Conserved HxD-histidine in the Catalytic Core of Protein Kinases | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

