Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04ICN
|
||||
| Former ID |
DIB010768
|
||||
| Drug Name |
DuP-630
|
||||
| Indication | Dermatitis [ICD9: 692.9; ICD10:L20-L30] | Terminated | [545582] | ||
| Company |
Bristol-Myers Squibb Pharma Co
|
||||
| Structure |
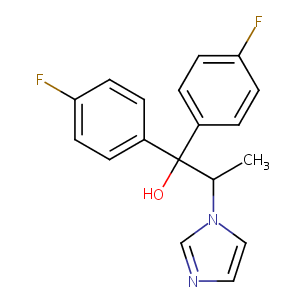
|
Download2D MOL |
|||
| Canonical SMILES |
C(C(n1cncc1)C)(c1ccc(cc1)F)(c1ccc(cc1)F)O
|
||||
| CAS Number |
CAS 128104-27-8
|
||||
| Target and Pathway | |||||
| Target(s) | Cytochrome P450 reductase | Target Info | Modulator | [550870] | |
| WikiPathways | Oxidation by Cytochrome P450 | ||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

