Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D04OSE
|
||||
| Former ID |
DAP000529
|
||||
| Drug Name |
Nifedipine
|
||||
| Synonyms |
Adalat; Adalate; Adapine; Adapress; Afeditab; Alat; Aldipin; Alfadal; Alonix; Angipec; Anifed; Anpine; Aprical; Bonacid; Calcibloc; Calcigard; Calcilat; Camont; Cardifen; Cardilat; Cardionorm; Chronadalate; Citilat; Coracten; Coral; Cordafen; Cordaflex; Cordalat; Cordicant; Cordilan; Cordipin; Cordipine; Corinfar; Corotrend; Corynphar; Depin; Dignokonstant; Dilafed; Dipinkor; Duranifin; Ecodipi; Ecodipin; Emaberin; Fedcor; Fenamon; Fenigidin; Fenihidin; Fenihidine; Glopir; Hadipin; Hexadilat; Infedipin; Introcar; Kordafen; Korinfar; Macorel; Megalat; Myogard; Nedipin; Nicardia; Nifangin; Nifar; Nifdemin; Nifebene; Nifecard; Nifecor; Nifedepat; Nifediac; Nifedical; Nifedicor; Nifedin; Nifedine; Nifedipino; Nifedipinum; Nifedipres; Nifelan; Nifelat; Nifelate; Nificard; Nifidine; Nifipen; Niphedipine; Orix; Oxcord; Pidilat; Procardia; Sepamit; Tibricol; Vascard; Zenusin; AWD Pharma Brand of Nifedipine; Adalat CC; Adalat CR; Adalat Crono; Adalat FT; Adalat GITS; Adalat LA; Adalat LP; Adalat Oros; Adalat PA; Adalat Retard; Adalat XL; Adalate LP; Adcock Ingram Brand of Nifedipine; Adipine XL; Afeditab CR; Alonix S; Aprical long; Bayer Brand of Nifedipine; Chronadalate LP; Coracten XL; Ecodipin E; Fedcor Retard; Fenamon SR; Fortipine LA; KRKA Brand of Nifedipine; Nifedical XL; Nifedipine Bayer Brand; Nifedipine GTIS; Nifedipine KRKA Brand; Nifedipine Monohydrochloride; Nifedipine Orion Brand; Nifedipine Pfizer Brand; Nifedipine Retard; Nifedirex LP; Nifelat Q; Nifensar XL; Orion Brand of Nifedipine; Pfizer Brand of Nifedipine; Procardia XL; Slofedipine XL; Tensipine MR; Adalat 10; Adalat 20; Adalat 5; Adalat GITS 30; Bay1040; N 7634; N1fedilat; Adalat (TN); Afeditab CR (TN); Alpha-Nifedipine Retard;Apo-Nifed; Bay-1040; KB-1712P; Monohydrochloride, Nifedipine; Nifedical (TN); Nifedipine-GTIS; Nifedipino [INN-Spanish]; Nifedipinum [INN-Latin]; Procardia (TN); Bay-a-1040; Nifedipine (JP15/USP/INN); Nifedipine [USAN:BAN:INN:JAN]
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Analgesics
|
||||
| Company |
Bayer
|
||||
| Structure |
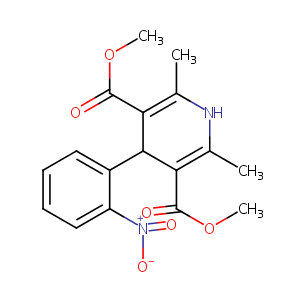
|
Download2D MOL |
|||
| Formula |
C17H18N2O6
|
||||
| InChI |
InChI=1S/C17H18N2O6/c1-9-13(16(20)24-3)15(14(10(2)18-9)17(21)25-4)11-7-5-6-8-12(11)19(22)23/h5-8,15,18H,1-4H3
|
||||
| InChIKey |
HYIMSNHJOBLJNT-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 21829-25-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9475, 634276, 841058, 855613, 3153213, 5010834, 6300547, 7847503, 7980124, 8145946, 8149443, 8152762, 10321407, 10513802, 11111548, 11111549, 11113899, 11119993, 11120481, 11120969, 11121456, 11121936, 11147076, 11335488, 11360727, 11362525, 11363873, 11364812, 11365087, 11366435, 11367374, 11367649, 11368997, 11369936, 11370321, 11370322, 11371671, 11372976, 11373250, 11373766, 11375536, 11375811, 11377159, 11378103, 11461699, 11466091, 11467211, 11484752, 11485902, 11488874
|
||||
| ChEBI ID |
ChEBI:7565
|
||||
| SuperDrug ATC ID |
C08CA05
|
||||
| SuperDrug CAS ID |
cas=021829254
|
||||
| Target and Pathway | |||||
| Target(s) | L-type calcium channel | Target Info | Modulator | [556264] | |
| References | |||||
| Ref 535549 | Are sildenafil and theophylline effective in the prevention of high-altitude pulmonary edema? Med Hypotheses. 2002 Aug;59(2):223-5. | ||||
| Ref 539636 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 2514). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

