| Drug General Information |
| Drug ID |
D07ZNR
|
| Former ID |
DNC011674
|
| Drug Name |
2,3,5,6-Tetrafluoro-4-pentafluorophenylazo-phenol
|
| Drug Type |
Small molecular drug
|
| Indication |
Discovery agent
|
Investigative |
[1]
|
|---|
| Structure |
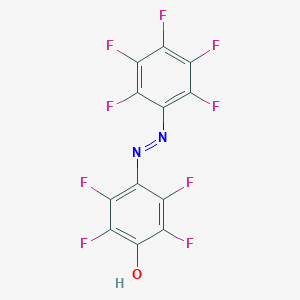
|
Download
2D MOL
3D MOL
|
| Formula |
C12HF9N2O
|
| Canonical SMILES |
C1(=C(C(=C(C(=C1F)F)F)F)F)NN=C2C(=C(C(=O)C(=C2F)F)F)F
|
| InChI |
1S/C12HF9N2O/c13-1-2(14)4(16)10(5(17)3(1)15)22-23-11-6(18)8(20)12(24)9(21)7(11)19/h22H
|
| InChIKey |
FNPGFLBPXIANTH-UHFFFAOYSA-N
|
| PubChem Compound ID |
|
| Target and Pathway |
| Target(s) |
17 alpha-hydroxylase-C17, 20-lyase |
Target Info |
Inhibitor |
[1]
|
|---|
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 |
Target Info |
Inhibitor |
[1]
|
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 |
Target Info |
Inhibitor |
[1]
|
|
BioCyc Pathway
|
Superpathway of steroid hormone biosynthesis
|
|
Glucocorticoid biosynthesis
|
|
Androgen biosynthesisPWY-7305:Superpathway of steroid hormone biosynthesis
|
|
Allopregnanolone biosynthesis
|
|
Androgen biosynthesis
|
|
KEGG Pathway
|
Steroid hormone biosynthesis
|
|
Metabolic pathways
|
|
Ovarian steroidogenesis
|
|
Prolactin signaling pathwayhsa00140:Steroid hormone biosynthesis
|
|
Prostate cancerhsa00140:Steroid hormone biosynthesis
|
|
NetPath Pathway
|
IL2 Signaling Pathway
|
|
PathWhiz Pathway
|
Androgen and Estrogen Metabolism
|
|
SteroidogenesisPW000045:Androgen and Estrogen Metabolism
|
|
Reactome
|
Androgen biosynthesis
|
|
Glucocorticoid biosynthesis
|
|
Endogenous sterolsR-HSA-193048:Androgen biosynthesisR-HSA-193048:Androgen biosynthesis
|
|
WikiPathways
|
Metapathway biotransformation
|
|
Steroid Biosynthesis
|
|
Oxidation by Cytochrome P450
|
|
Metabolism of steroid hormones and vitamin D
|
|
Glucocorticoid & Mineralcorticoid Metabolism
|
|
Prostate Cancer
|
|
Phase 1 - Functionalization of compounds
|
| References |
| REF 1 | J Med Chem. 1990 Sep;33(9):2452-5.Hydroxyperfluoroazobenzenes: novel inhibitors of enzymes of androgen biosynthesis. |
|---|