Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0B1YW
|
||||
| Former ID |
DNC003644
|
||||
| Drug Name |
8-Phenyl-1-propyl-3,7-dihydro-purine-2,6-dione
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [533940] | ||
| Structure |
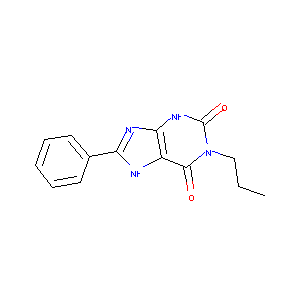
|
Download2D MOL |
|||
| Formula |
C14H14N4O2
|
||||
| Canonical SMILES |
CCCN1C(=O)C2=C(NC1=O)N=C(N2)C3=CC=CC=C3
|
||||
| InChI |
1S/C14H14N4O2/c1-2-8-18-13(19)10-12(17-14(18)20)16-11(15-10)9-6-4-3-5-7-9/h3-7H,2,8H2,1H3,(H,15,16)(H,17,20)
|
||||
| InChIKey |
LNATVXLECNOWHK-UHFFFAOYSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Adenosine A2b receptor | Target Info | Inhibitor | [526958] | |
| Adenosine A2a receptor | Target Info | Inhibitor | [526958] | ||
| Adenosine A1 receptor | Target Info | Inhibitor | [533940] | ||
| KEGG Pathway | Rap1 signaling pathway | ||||
| Calcium signaling pathway | |||||
| Neuroactive ligand-receptor interaction | |||||
| Vascular smooth muscle contraction | |||||
| Alcoholismhsa04015:Rap1 signaling pathway | |||||
| cAMP signaling pathway | |||||
| Parkinson's disease | |||||
| Alcoholismhsa04022:cGMP-PKG signaling pathway | |||||
| Sphingolipid signaling pathway | |||||
| Morphine addiction | |||||
| Pathway Interaction Database | C-MYB transcription factor networkhif2pathway:HIF-2-alpha transcription factor network | ||||
| References | |||||
| Ref 526958 | J Med Chem. 2004 Feb 12;47(4):1031-43.Preparation, properties, reactions, and adenosine receptor affinities of sulfophenylxanthine nitrophenyl esters: toward the development of sulfonic acid prodrugswith peroral bioavailability. | ||||
| Ref 533940 | J Med Chem. 1993 Oct 29;36(22):3341-9.Synthesis of paraxanthine analogs (1,7-disubstituted xanthines) and other xanthines unsubstituted at the 3-position: structure-activity relationships at adenosine receptors. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

