Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0ER2C
|
||||
| Former ID |
DIB010821
|
||||
| Drug Name |
Triptoreline
|
||||
| Synonyms |
Triptoreline (microsphere depot formulation, prostate cancer); Triptoreline (microsphere depot formulation, prostate cancer), GP Pharm; Triptoreline (one-month sustained release formulation, prostate cancer), GP Pharm; Triptoreline (three-month sustained release formulation, prostate cancer), GP Pharm
|
||||
| Indication | Prostate cancer [ICD9: 185; ICD10:C61] | Phase 4 | [522253] | ||
| Company |
GP Pharm SA
|
||||
| Structure |
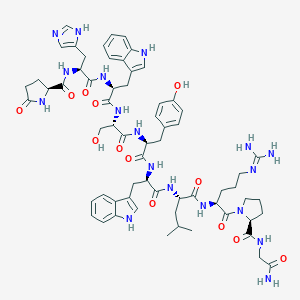
|
Download2D MOL |
|||
| Formula |
C64H82N18O13
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | Gonadotropin-releasing hormone receptor | Target Info | Modulator | [528589] | |
| NetPath Pathway | IL1 Signaling Pathway | ||||
| IL2 Signaling Pathway | |||||
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

