Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0H0HJ
|
||||
| Former ID |
DAP000081
|
||||
| Drug Name |
Tranylcypromine
|
||||
| Synonyms |
GJZ; Jatrosom; Parnate; Parnitene; Tranilcipromina; Transamine; Tranylcyprominum; Allphar Brand of Tranylcypromine Sulfate; Esparma Brand of Tranylcypromine Sulfate; GlaxoSmithKline Brand of Tranylcypromine Sulfate; Goldshield Brand of Tranylcypromine Sulfate; Link Brand of Tranylcypromine Sulfate; Racemic Tranylcypromine; SmithKline Brand of Tranylcypromine Sulfate; SKF 385; Trans 2 Phenylcyclopropylamine; D-Tranylcypromine; Dl-Tranylcypromine; Jatrosom (TN); L-Tranylcypromine; Parmodalin (TN); Parnate (TN); Parstelin (TN); SKF Trans-385; Sicoton (TN); Sulfate, Tranylcypromine; Tranilcipromina [INN-Spanish]; Transamin (TN); Transamine (TN); Transapin (TN); Tranylcypromine (INN); Tranylcypromine [INN:BAN]; Tranylcyprominum [INN-Latin]; Tylciprine (TN); Trans-2-Phenylcyclopropylamine; Trans-DL-2-Phenylcyclopropylamine; Trans-(-)-2-Phenylcyclopropanamine; Cyclopropanamine, 2-phenyl-, (1R-trans)-(9CI); (+)-(R)-Tranylcypromine; (+)-Tranylcypromine; (+)-trans-2-Phenylcyclopropylamine; (-)-Tranylcypromine; (1R)-2-phenylcyclopropan-1-amine; (1R,2R)-2-phenylcyclopropan-1-amine; (1R,2S)-2-phenylcyclopropan-1-amine; (1R,2S)-2-phenylcyclopropanamine; (1S)-2-phenylcyclopropan-1-amine; (1S,2R)-2-phenylcyclopropan-1-amine; (1S,2S)-2-phenylcyclopropan-1-amine; (2S)-2-phenylcyclopropan-1-amine; 2-Phenylcyclopropanamine; 2-phenylcyclopropan-1-amine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Major depressive episode without melancholia [ICD10:F30-F39] | Approved | [551871] | ||
| Therapeutic Class |
Antidepressants
|
||||
| Company |
GlaxoSmithKline plc
|
||||
| Structure |
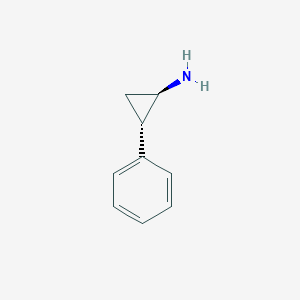
|
Download2D MOL |
|||
| Formula |
C9H11N
|
||||
| InChI |
InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2
|
||||
| InChIKey |
AELCINSCMGFISI-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 155-09-9
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
120008, 5659228, 8153403, 11364492, 11367054, 11369616, 11371975, 11374796, 11377778, 11490883, 11493033, 11495412, 29224572, 29577064, 47662866, 48259831, 49959880, 50111217, 50359559, 57322828, 90340789, 103121804, 103285851, 104008102, 104309494, 117357576, 117530064, 124451899, 124638213, 124881215, 125335618, 125572982, 125811258, 127394661, 131380368, 137051941, 142491745, 143452884, 162534986, 164319473, 166461410, 174006850, 176317175, 177343471, 178101964, 179039123, 223711656, 224089771, 226425849, 241093276
|
||||
| SuperDrug ATC ID |
N06AF04
|
||||
| SuperDrug CAS ID |
cas=000155099
|
||||
| Target and Pathway | |||||
| Target(s) | Amine oxidase [flavin-containing] A | Target Info | Inhibitor | [536265], [537141] | |
| Amine oxidase [flavin-containing] B | Target Info | Inhibitor | [536265], [537851] | ||
| BioCyc Pathway | Superpathway of tryptophan utilization | ||||
| Dopamine degradation | |||||
| Putrescine degradation III | |||||
| Noradrenaline and adrenaline degradation | |||||
| Serotonin degradation | |||||
| Superpathway of melatonin degradation | |||||
| Melatonin degradation IIPWY66-401:Superpathway of tryptophan utilization | |||||
| Tryptophan degradation via tryptamine | |||||
| KEGG Pathway | Glycine, serine and threonine metabolism | ||||
| Arginine and proline metabolism | |||||
| Histidine metabolism | |||||
| Tyrosine metabolism | |||||
| Phenylalanine metabolism | |||||
| Tryptophan metabolism | |||||
| Drug metabolism - cytochrome P450 | |||||
| Metabolic pathways | |||||
| Serotonergic synapse | |||||
| Dopaminergic synapse | |||||
| Cocaine addiction | |||||
| Amphetamine addiction | |||||
| Alcoholismhsa00260:Glycine, serine and threonine metabolism | |||||
| Alcoholism | |||||
| NetPath Pathway | IL4 Signaling Pathway | ||||
| Pathway Interaction Database | Alpha-synuclein signaling | ||||
| References | |||||
| Ref 536265 | Tranylcypromine: new perspectives on an "old" drug. Eur Arch Psychiatry Clin Neurosci. 2006 Aug;256(5):268-73. | ||||
| Ref 537141 | Tramadol and another atypical opioid meperidine have exaggerated serotonin syndrome behavioural effects, but decreased analgesic effects, in genetically deficient serotonin transporter (SERT) mice. Int J Neuropsychopharmacol. 2009 Mar 11:1-11. | ||||
| Ref 537851 | Dopamine D2 receptors: a potential pharmacological target for nomifensine and tranylcypromine but not other antidepressant treatments. Pharmacol Biochem Behav. 1995 Aug;51(4):565-9. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

