Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0ML1F
|
||||
| Former ID |
DAP000060
|
||||
| Drug Name |
Adefovir Dipivoxil
|
||||
| Synonyms |
Adefovirdipivoxl; Hepsera; Preveon; YouHeDing; Adefovir depivoxil; Adefovir pivoxil; GS 0840; GS 840;Piv2PMEA; Adefovir dipivoxil (USAN); Adefovir pivoxil (JAN); Bis(pom)PMEA; Bis-POM PMEA; GS-0840; GS-840; Hepsera (TM); Hepsera (TN); Preveon (TN); Bis(POM)-PMEA; Bis-POM PMEA, Adefovir Dipivoxil; Bis-POM PMEA, Preveon, Hepsera, Adefovir Dipivoxil; Bis(pivaloyloxymethyl)-9-(2-phosphonylmethoxyethyl)adenine; [2-(6-aminopurin-9-yl)ethoxymethyl-(2,2-dimethylpropanoyloxymethoxy)phosphoryl]oxymethyl 2,2-dimethylpropanoate; Propanoic acid,2,2-dimethyl-(((2-(6-amino-9H-purin-9-yl)ehtoxy)mehtyl)phosphinyldiene)bis(oxymehtylene)ester; Propanoic acid, 2,2-dimethyl-, (((2-(6-amino-9H-purin-9-yl)ethoxy)methyl)phosphinylidene)bis(oxymethylene) ester; (((2-(6-Amino-9H-purin-9-yl)ethoxy)methyl)phosphinylidene)bis(oxymethylene) 2,2-dimethylpropanoate; ((2-(6-Amino-9H-purin-9-yl)ethoxy)methyl)phosphonic acid, diester with hydroxymethyl pivalate; 9-(2-((-Bis((pivaloyloxy)methoxy)phosphinyl)methoxy)ethyl)adenine; 9-(2-((Bis((pivaloyloxy)methoxy)phosphinyl)methoxy)ethyl)adenine; ADV
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiviral Agents
|
||||
| Company |
Gilead Sciences
|
||||
| Structure |
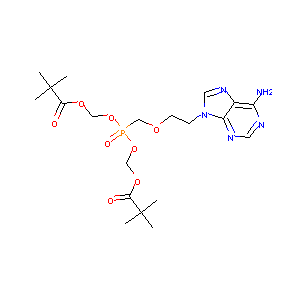
|
Download2D MOL |
|||
| Formula |
C20H32N5O8P
|
||||
| Canonical SMILES |
CC(C)(C)C(=O)OCOP(=O)(COCCN1C=NC2=C1N=CN=C2N)OCOC(=O)C(<br />C)(C)C
|
||||
| InChI |
1S/C20H32N5O8P/c1-19(2,3)17(26)30-11-32-34(28,33-12-31-18(27)20(4,5)6)13-29-8-7-25-10-24-14-15(21)22-9-23-16(14)25/h9-10H,7-8,11-13H2,1-6H3,(H2,21,22,23)
|
||||
| InChIKey |
WOZSCQDILHKSGG-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 142340-99-6
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
612309, 7848718, 8187107, 11528768, 12014790, 14908863, 26758046, 43118206, 46507520, 49830940, 57314164, 71824987, 74485826, 87244050, 87245424, 92712536, 103240599, 104253333, 104321855, 109692937, 118048871, 119525323, 124757452, 125164256, 125340236, 126592990, 126630083, 126656634, 126665844, 129613204, 131298815, 134222429, 134337388, 134338819, 134340371, 135018282, 135565514, 135692243, 135727110, 136345656, 136368131, 136946538, 136949103, 137006009, 142181502, 143493341, 144115540, 144180310, 144205796, 152242352
|
||||
| SuperDrug ATC ID |
J05AF08
|
||||
| SuperDrug CAS ID |
cas=142340996
|
||||
| Drug Resistance Mutation (DRM) | |||||
| DRM | DRM Info | ||||
| Target and Pathway | |||||
| Target(s) | HIV reverse transcriptase | Target Info | Modulator | [556264] | |
| References | |||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

