Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Q2MH
|
||||
| Former ID |
DAP000963
|
||||
| Drug Name |
Hexafluronium bromide
|
||||
| Synonyms |
Hexafluorenium; Milaxen; Mylaxen; Bromuro de hexafluronio; Esafluronio bromuro; Esafluronio bromuro [DCIT]; Hexafluorenium bromide; Hexafluorenium bromide [USAN]; Hexafluorenium dibromide; Hexafluorenium dibromide salt; Hexafluronii bromidum; Bromure d'hexafluronium; Bromuro de hexafluronio [INN-Spanish]; Hexafluorenium bromide (USAN); Hexafluronii bromidum [INN-Latin]; Hexafluronium bromide (INN); IN-117; Mylaxen (TN); Bromure d'hexafluronium [INN-French]; Hexamethylene bis(9-fluorenyldimethylammonium)dibromide; Hexamethylenebis(9-fluorenyldimethylammonium bromide); Hexamethylenebis(dimethyl-9-fluorenylammonium bromide); Hexamethylenebis(fluoren-9-yldimethylammonium) dibromide; AMMONIUM, HEXAMETHYLENEBIS(FLUOREN-9-YLDIMETHYL-, DIBROMIDE; Ammonium, hexamethylenebis(fluoren-9-yldimethyl-, bromide; N,N'-Di(9-fluorenyl)-N,N,N',N'-tetramethyly-hexamethylendi(ammoniumbromid); 1,6-Bis(9 fluorenyldimethyl-ammonium)hexane bromide; 1,6-Hexanediaminium, N,N'-di-9H-fluoren-9-yl-N,N,N',N'-tetramethyl-, dibromide; 9H-fluoren-9-yl-[6-[9H-fluoren-9-yl(dimethyl)azaniumyl]hexyl]-dimethylazanium dibromide
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Spasms; Pain [ICD9: 338,780; ICD10:R52, G89] | Approved | [1], [2] | ||
| Therapeutic Class |
Analgesics
|
||||
| Structure |
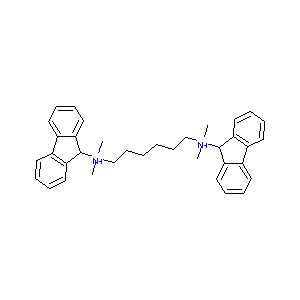
|
Download2D MOL |
|||
| Formula |
C36H42Br2N2
|
||||
| Canonical SMILES |
C[N+](C)(CCCCCC[N+](C)(C)C1C2=CC=CC=C2C3=CC=CC=C13)C4C5<br />=CC=CC=C5C6=CC=CC=C46.[Br-].[Br-]
|
||||
| InChI |
1S/C36H42N2.2BrH/c1-37(2,35-31-21-11-7-17-27(31)28-18-8-12-22-32(28)35)25-15-5-6-16-26-38(3,4)36-33-23-13-9-19-29(33)30-20-10-14-24-34(30)36;;/h7-14,17-24,35-36H,5-6,15-16,25-26H2,1-4H3;2*1H/q+2;;/p-2
|
||||
| InChIKey |
WDEFPRUEZRUYNW-UHFFFAOYSA-L
|
||||
| CAS Number |
CAS 317-52-2
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
M03AC05
|
||||
| Target and Pathway | |||||
| Target(s) | Cholinesterase | Target Info | Inhibitor | [3] | |
| PANTHER Pathway | Muscarinic acetylcholine receptor 1 and 3 signaling pathway | ||||
| Muscarinic acetylcholine receptor 2 and 4 signaling pathway | |||||
| Nicotinic acetylcholine receptor signaling pathway | |||||
| WikiPathways | Irinotecan Pathway | ||||
| References | |||||
| REF 1 | Pharmacology, clinical uses, and adverse effects of ceruletide, a cholecystokinetic agent. Pharmacotherapy. 1982 Jul-Aug;2(4):223-34. | ||||
| REF 2 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| REF 3 | Synergistic effect of acidosis and succinylcholine-induced hyperkalemia in spinal cord transected rats. Acta Anaesthesiol Scand. 1984 Feb;28(1):87-90. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

