Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D00RJJ
|
||||
| Former ID |
DAP001386
|
||||
| Drug Name |
Ganirelix
|
||||
| Synonyms |
Ganirelixum; Orgalutran; Ganirelix acetate; Antagon (TN); Ganirelix (INN); Ganirelix [INN:BAN]; Ganirelixum [INN-Latin]; Orgalutran (TN); RS-26306
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Ovulation [ICD9: 256; ICD10:E28] | Approved | [1], [2] | ||
| Therapeutic Class |
Abortifacient Agents
|
||||
| Company |
Organon International
|
||||
| Structure |
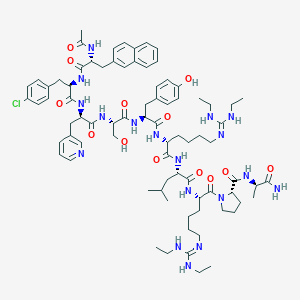
|
Download2D MOL |
|||
| Formula |
C80H113ClN18O13
|
||||
| InChI |
InChI=1S/C80H113ClN18O13/c1-9-84-79(85-10-2)88-38-17-15-24-60(70(104)94-62(41-49(5)6)71(105)93-61(25-16-18-39-89-80(86-11-3)87-12-4)78(112)99-40-20-26-68(99)77(111)90-50(7)69(82)103)92-73(107)64(44-53-30-35-59(102)36-31-53)97-76(110)67(48-100)98-75(109)66(46-55-21-19-37-83-47-55)96-74(108)65(43-52-28-33-58(81)34-29-52)95-72(106)63(91-51(8)101)45-54-27-32-56-22-13-14-23-57(56)42-54/h13-14,19,21-23,27-37,42,47,49-50,60-68,100,102H,9-12,15-18,20,24-26,38-41,43-46,48H2,1-8H3,(H2,82,103)(H,90,111)(H,91,101)(H,92,107)(H,93,105)(H,94,104)(H,95,106)(H,96,108)(H,97,110)(H,98,109)(H2,84,85,88)(H2,86,87,89)/t50-,60-,61+,62+,63?,64-,65+,66+,67-,68?/m1/s1
|
||||
| InChIKey |
GJNXBNATEDXMAK-RXXWGPEVSA-N
|
||||
| CAS Number |
CAS 123246-29-7
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID | |||||
| SuperDrug ATC ID |
H01CC01
|
||||
| SuperDrug CAS ID |
cas=124904934
|
||||
| Target and Pathway | |||||
| Target(s) | Gonadotropin-releasing hormone receptor | Target Info | Antagonist | [3] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| GnRH signaling pathway | |||||
| NetPath Pathway | IL1 Signaling Pathway | ||||
| IL2 Signaling Pathway | |||||
| PANTHER Pathway | Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | ||||
| Reactome | Hormone ligand-binding receptors | ||||
| G alpha (q) signalling events | |||||
| WikiPathways | Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Peptide GPCRs | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| GPCRs, Other | |||||
| References | |||||
| REF 1 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. Epub 2007 Feb 20. | ||||
| REF 2 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 3877). | ||||
| REF 3 | Age attenuates testosterone secretion driven by amplitude-varying pulses of recombinant human luteinizing hormone during acute gonadotrope inhibition in healthy men. J Clin Endocrinol Metab. 2007 Sep;92(9):3626-32. Epub 2007 Jun 19. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

