Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D08FKH
|
||||
| Former ID |
DIB014322
|
||||
| Drug Name |
KLH-2109
|
||||
| Indication | Endometriosis [ICD9: 617; ICD10:N80] | Phase 2 | [1] | ||
| Company |
Kissei Pharmaceutical Co Ltd
|
||||
| Structure |
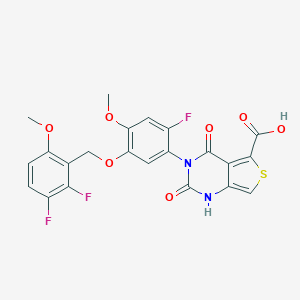
|
Download2D MOL |
|||
| Target and Pathway | |||||
| Target(s) | Gonadotropin-releasing hormone receptor | Target Info | Agonist | [2] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| GnRH signaling pathway | |||||
| NetPath Pathway | IL1 Signaling Pathway | ||||
| IL2 Signaling Pathway | |||||
| PANTHER Pathway | Heterotrimeric G-protein signaling pathway-Gq alpha and Go alpha mediated pathway | ||||
| Reactome | Hormone ligand-binding receptors | ||||
| G alpha (q) signalling events | |||||
| WikiPathways | Gastrin-CREB signalling pathway via PKC and MAPK | ||||
| Peptide GPCRs | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| GPCRs, Other | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT01533532) A Randomized, Placebo-controlled, Double-blind Study of KLH-2109 in Patients With Endometriosis (2). U.S. National Institutes of Health. | ||||
| REF 2 | Clinical pipeline report, company report or official report of Avarx. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

