Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0E2IN
|
||||
| Former ID |
DIB002021
|
||||
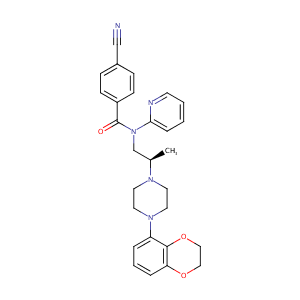
| Drug Name |
LECOZOTAN HYDROCHLORIDE
|
||||
| Synonyms |
Lecozotan hydrochloride < USAN; Prop INNM >; SRA-333; 4-Cyano-N-[2(R)-[4-(2,3-dihydro-1,4-benzodioxin-5-yl)piperazin-1-yl]propyl]-N-(2-pyridyl)benzamide hydrochloride
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Cognitive disorders [ICD9: 290-294, 294.0, 780.09, 780.9, 780.93; ICD10:F01-F07, F04, F05, R41.3] | Phase 2/3 | [1] | ||
| Structure |
|
Download2D MOL |
|||
| Formula |
C28H30ClN5O3
|
||||
| Canonical SMILES |
C[C@H](CN(C(=O)c1ccc(cc1)C#N)c2ccccn2)N3CCN(CC3)c4cccc5<br />OCCOc45
|
||||
| InChI |
1S/C28H29N5O3/c1-21(31-13-15-32(16-14-31)24-5-4-6-25-27(24)36-18-17-35-25)20-33(26-7-2-3-12-30-26)28(34)23-10-8-22(19-29)9-11-23/h2-12,21H,13-18,20H2,1H3/t21-/m1/s1
|
||||
| InChIKey |
NRPQELCNMADTOZ-OAQYLSRUSA-N
|
||||
| PubChem Compound ID | |||||
| Target and Pathway | |||||
| Target(s) | 5-hydroxytryptamine 1A receptor | Target Info | Antagonist | [2], [3] | |
| KEGG Pathway | cAMP signaling pathway | ||||
| Neuroactive ligand-receptor interaction | |||||
| Serotonergic synapse | |||||
| PANTHER Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | ||||
| 5HT1 type receptor mediated signaling pathway | |||||
| Reactome | Serotonin receptors | ||||
| G alpha (i) signalling events | |||||
| WikiPathways | Serotonin HTR1 Group and FOS Pathway | ||||
| SIDS Susceptibility Pathways | |||||
| Monoamine GPCRs | |||||
| GPCRs, Class A Rhodopsin-like | |||||
| GPCR ligand binding | |||||
| GPCR downstream signaling | |||||
| References | |||||
| REF 1 | ClinicalTrials.gov (NCT00277810) Study Evaluating the Safety, Tolerability, and Efficacy of Lecozotan SR in Outpatients With Alzheimer's Disease. U.S. National Institutes of Health. | ||||
| REF 2 | A positron emission tomography study to assess binding of lecozotan, a novel 5-hydroxytryptamine-1A silent antagonist, to brain 5-HT1A receptors in healthy young and elderly subjects, and in patientswith Alzheimer's disease. Clin Pharmacol Ther. 2008 Jan;83(1):86-96. Epub 2007 May 16. | ||||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

