Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0F0YZ
|
||||
| Former ID |
DAP000671
|
||||
| Drug Name |
Pentobarbital
|
||||
| Synonyms |
Aethaminalum; Dorsital; Etaminal; Ethaminal; Mebubarbital; Mebumal; Nebralin; Nembutal; Neodorm; Pentabarbital; Pentabarbitone; Pentobarbitale; Pentobarbitalum; Pentobarbitone; Pentobarbiturate; Rivadorm; Pentobarbital calcium; Pentobarbital suppository dosage form; Pentobarbitale [DCIT]; Pentobarbituric acid; Ethyl-propylmethylcarbinylbarbituric acid; Nembutal (TN); Nembutal (VAN); Neodorm (VAN); Neodorm (new); Pentobarbital (VAN); Pentobarbital [USP:INN]; Pentobarbital, Monosodium Salt; Pentobarbitalum [INN-Latin]; Pentobarbitone (VAN); Pentobarbital (USP/INN); (+-)-5-Ethyl-5-(1-methylbutyl)barbituric acid; 5-Ethyl-5-(1-methylbutyl)-2,4,6(1H,3H,5H)-pyrimidinetrione; 5-Ethyl-5-(1-methylbutyl)barbituric acid; 5-Ethyl-5-(1-methylbutyl)malonylurea; 5-Ethyl-5-(sec-pentyl)barbituric acid; 5-ethyl-2-hydroxy-5-(1-methylbutyl)pyrimidine-4,6(1H,5H)-dione; 5-ethyl-5-(pentan-2-yl)pyrimidine-2,4,6(1H,3H,5H)-trione;5-ethyl-5-pentan-2-yl-1,3-diazinane-2,4,6-trione
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Insomnia; Anxiety disorder [ICD9: 307.41, 307.42, 327.0, 780.51, 780.52, 300, 311; ICD10:F51.0, G47.0, F32, F40-F42] | Approved | [1], [2], [3] | ||
| Therapeutic Class |
Hypnotics and Sedatives
|
||||
| Company |
Oak Pharmaceuticals Inc
|
||||
| Structure |
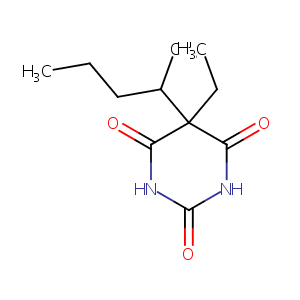
|
Download2D MOL |
|||
| Formula |
C11H18N2O3
|
||||
| InChI |
InChI=1S/C11H18N2O3/c1-4-6-7(3)11(5-2)8(14)12-10(16)13-9(11)15/h7H,4-6H2,1-3H3,(H2,12,13,14,15,16)
|
||||
| InChIKey |
WEXRUCMBJFQVBZ-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 76-74-4
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9626, 88629, 4875675, 7687719, 7847565, 8150116, 8152908, 10535336, 11336167, 11361406, 11364680, 11367242, 11369804, 11372904, 11374147, 11377967, 11462378, 11485937, 11489807, 11491598, 11492389, 11495597, 11533361, 15196098, 26612988, 26679656, 26750091, 29223823, 46508399, 47291210, 47440355, 47813402, 47885494, 48187638, 48416394, 49874341, 50107805, 53788844, 56313777, 57322422, 79087870, 92308182, 99302080, 103165831, 103858250, 103952389, 104307283, 104826284, 117425382, 124637312
|
||||
| ChEBI ID |
ChEBI:7983
|
||||
| SuperDrug ATC ID |
N05CA01
|
||||
| SuperDrug CAS ID |
cas=000076744
|
||||
| Target and Pathway | |||||
| Target(s) | Gamma-aminobutyric acid receptor subunit alpha-1 | Target Info | Antagonist | [4], [5] | |
| KEGG Pathway | Neuroactive ligand-receptor interaction | ||||
| Retrograde endocannabinoid signaling | |||||
| GABAergic synapse | |||||
| Morphine addiction | |||||
| Nicotine addiction | |||||
| Reactome | Ligand-gated ion channel transport | ||||
| GABA A receptor activation | |||||
| WikiPathways | SIDS Susceptibility Pathways | ||||
| Neurotransmitter Receptor Binding And Downstream Transmission In The Postsynaptic Cell | |||||
| Iron uptake and transport | |||||
| References | |||||
| REF 1 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 083270. | ||||
| REF 2 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5480). | ||||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015 | ||||
| REF 4 | DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901-6. Epub 2007 Nov 29. | ||||
| REF 5 | Effect of barbiturates on polyphosphoinositide biosynthesis and protein kinase C activity in synaptosomes. Neuropharmacology. 1989 Dec;28(12):1317-23. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

