Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0RF2M
|
||||
| Former ID |
DNC005811
|
||||
| Drug Name |
NIPECOTIC ACID
|
||||
| Drug Type |
Small molecular drug
|
||||
| Indication | Discovery agent | Investigative | [1] | ||
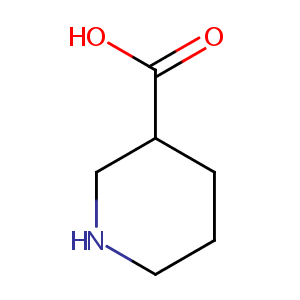
| Structure |
|
Download2D MOL |
|||
| Formula |
C6H11NO2
|
||||
| InChI |
InChI=1S/C6H11NO2/c8-6(9)5-2-1-3-7-4-5/h5,7H,1-4H2,(H,8,9)
|
||||
| InChIKey |
XJLSEXAGTJCILF-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 498-95-3
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
3138351, 7404102, 8139863, 8150178, 8152771, 11335323, 11360562, 11363247, 11365809, 11368371, 11372643, 11373796, 11376533, 11461534, 11466978, 11468098, 11484963, 11486815, 11489000, 11491527, 11491984, 11494167, 15170656, 17404605, 24278161, 26613203, 26679237, 26747103, 29223592, 35033299, 47213193, 47216644, 47440115, 47440116, 47736328, 47810612, 47810613, 47885272, 48034968, 48110317, 48184862, 49698820, 49833219, 50015592, 50104354, 50104355, 50104356, 50396592, 53777147, 57322300
|
||||
| Target and Pathway | |||||
| Target(s) | Sodium- and chloride-dependent GABA transporter 1 | Target Info | Inhibitor | [2] | |
| GABA transporter-3 | Target Info | Inhibitor | [2] | ||
| 4-aminobutyrate aminotransferase, mitochondrial | Target Info | Inhibitor | [3] | ||
| Adenosine A3 receptor | Target Info | Inhibitor | [4] | ||
| Gamma-aminobutyric acid receptor | Target Info | Inhibitor | [5] | ||
| BioCyc Pathway | GABA shunt | ||||
| Valine degradation | |||||
| Beta-alanine degradation | |||||
| 4-aminobutyrate degradation | |||||
| KEGG Pathway | GABAergic synapsehsa00250:Alanine, aspartate and glutamate metabolism | ||||
| Valine, leucine and isoleucine degradation | |||||
| beta-Alanine metabolism | |||||
| Propanoate metabolism | |||||
| Butanoate metabolism | |||||
| Metabolic pathways | |||||
| GABAergic synapse | |||||
| PANTHER Pathway | Aminobutyrate degradation | ||||
| Pyrimidine Metabolism | |||||
| Gamma-aminobutyric acid synthesis | |||||
| PathWhiz Pathway | Aspartate Metabolism | ||||
| Glutamate Metabolism | |||||
| Beta-Alanine Metabolism | |||||
| Valine, Leucine and Isoleucine Degradation | |||||
| Propanoate Metabolism | |||||
| Reactome | Na+/Cl- dependent neurotransmitter transportersR-HSA-417973:Adenosine P1 receptors | ||||
| G alpha (i) signalling events | |||||
| WikiPathways | Monoamine Transport | ||||
| NRF2 pathwayWP2685:GABA synthesis, release, reuptake and degradation | |||||
| Alanine and aspartate metabolismWP80:Nucleotide GPCRs | |||||
| GPCRs, Class A Rhodopsin-like | |||||
| GPCRs, Other | |||||
| References | |||||
| REF 1 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 4564). | ||||
| REF 2 | J Med Chem. 1981 Jul;24(7):788-94.Epimeric cis-decahydroquinoline-5-carboxylic acids: effects on gamma-aminobutyric acid uptake and receptor binding in vitro. | ||||
| REF 3 | J Med Chem. 1982 Feb;25(2):113-6.Aminomethyl-1,2,4-benzothiadiazines as potential analogues of gamma-aminobutyric acid. Unexpected discovery of a taurine antagonist. | ||||
| REF 4 | J Med Chem. 2005 Nov 3;48(22):6887-96.2-n-Butyl-9-methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-ylamine and analogues as A2A adenosine receptor antagonists. Design, synthesis, and pharmacological characterization. | ||||
| REF 5 | J Med Chem. 1985 May;28(5):653-60.Orally active and potent inhibitors of gamma-aminobutyric acid uptake. | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

