Drug Information
| Drug General Information | |||||
|---|---|---|---|---|---|
| Drug ID |
D0Y0ER
|
||||
| Former ID |
DAP000315
|
||||
| Drug Name |
Thiethylperazine
|
||||
| Synonyms |
ETHYLTHIOPERAZINE; Theithylperazine; Thiethylperazinum; Thiethylpipezazine; Thioethylperazine; Tietilperazina; Torecan; Tietilperazina [DCIT]; Thiethylperazinum [INN-Latin]; Torecan (TN); Thiethylperazine (USAN/INN); Thiethylperazine [USAN:INN:BAN]; Norzine (*Dimaleate*); GS-95 (*Dimaleate*); 10H-Phenothiazine, 2-(ethylthio)-10-[3-(4-methyl-1-piperazinyl)propyl]-, (Z)-2-butenedioate (1:2); 2-(Ethylthio)-10-(3-(4-methyl-1-piperazinyl)propyl)phenothiazine; 2-(ethylsulfanyl)-10-[3-(4-methylpiperazin-1-yl)propyl]-10H-phenothiazine; 2-(ethylthio)-10-[3-(4-methylpiperazin-1-yl)propyl]-10H-phenothiazine; 2-ethylsulfanyl-10-[3-(4-methylpiperazin-1-yl)propyl]phenothiazine; 3-Ethylmercapto-10-(1'-methylpiperazinyl-4'-propyl)phenothiazine
|
||||
| Drug Type |
Small molecular drug
|
||||
| Therapeutic Class |
Antiemetics
|
||||
| Company |
Norvatis Phamaceuticals Corporation
|
||||
| Structure |
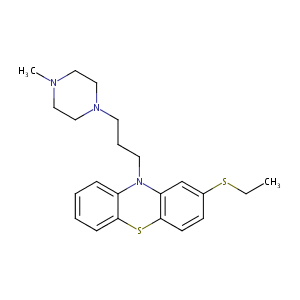
|
Download2D MOL |
|||
| Formula |
C22H29N3S2
|
||||
| InChI |
InChI=1S/C22H29N3S2/c1-3-26-18-9-10-22-20(17-18)25(19-7-4-5-8-21(19)27-22)12-6-11-24-15-13-23(2)14-16-24/h4-5,7-10,17H,3,6,11-16H2,1-2H3
|
||||
| InChIKey |
XCTYLCDETUVOIP-UHFFFAOYSA-N
|
||||
| CAS Number |
CAS 1420-55-9
|
||||
| PubChem Compound ID | |||||
| PubChem Substance ID |
9342, 618292, 5646731, 7849413, 7980773, 8153348, 10507857, 14879091, 29224490, 46506502, 48416610, 49699402, 50006536, 56352874, 57322781, 76368511, 85209578, 103462495, 104309248, 125089234, 125823178, 127339724, 127339725, 127339726, 134222490, 134337627, 134979014, 135739860, 137001505, 142971091, 160963719, 162939803, 178103879, 179116916, 184527123, 198985013, 226432954, 249932754, 251971089, 252655192
|
||||
| ChEBI ID |
ChEBI:9544
|
||||
| SuperDrug ATC ID |
R06AD03
|
||||
| SuperDrug CAS ID |
cas=001420559
|
||||
| Target and Pathway | |||||
| Target(s) | D(2) dopamine receptor | Target Info | Antagonist | [537675], [537780] | |
| References | |||||
| Ref 538460 | FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (NDA) 012753. | ||||
| Ref 542328 | (http://www.guidetopharmacology.org/) Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7306). | ||||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

