Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D0A4TC
|
|||
| Former ID |
DNC000955
|
|||
| Drug Name |
MMI270
|
|||
| Synonyms |
CGS-27023A; CGS-27023; UNII-80AXY59IT2; 80AXY59IT2; N-HYDROXY-2(R)-[[(4-METHOXYPHENYL)SULFONYL](3-PICOLYL)AMINO]-3-METHYLBUTANAMIDE HYDROCHLORIDE; CHEMBL514138; (2R)-N-hydroxy-2-[(4-methoxyphenyl)sulfonyl-(pyridin-3-ylmethyl)amino]-3-methylbutanamide; CGS; MMI270; 1eub; MMI270B free base; hydroxamate analogue 1; 2w0d; 1bm6; MMI-270B free base; AC1L9JQY; 3MP-HAV-MSB; CGS-27023A free base; BMCL16311 Compound 1a; BDBM8465; SCHEMBL3468445; GTPL8846; CHEMBL267178; BSIZUMJRKYHEBR-QGZVFWFLSA-N; CGS 27023; BDBM50066658; DB07556; 161314-70-1
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Discovery agent [ICD-11: N.A.] | Investigative | [1] | |
| Structure |
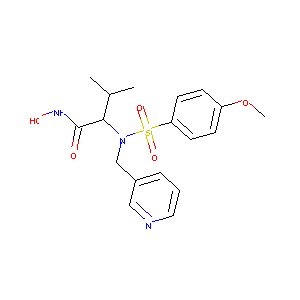 |
Download2D MOL |
||
| Formula |
C18H23N3O5S
|
|||
| Canonical SMILES |
CC(C)C(C(=O)NO)N(CC1=CN=CC=C1)S(=O)(=O)C2=CC=C(C=C2)OC
|
|||
| InChI |
1S/C18H23N3O5S/c1-13(2)17(18(22)20-23)21(12-14-5-4-10-19-11-14)27(24,25)16-8-6-15(26-3)7-9-16/h4-11,13,17,23H,12H2,1-3H3,(H,20,22)/t17-/m1/s1
|
|||
| InChIKey |
BSIZUMJRKYHEBR-QGZVFWFLSA-N
|
|||
| CAS Number |
CAS 161314-70-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
835264, 835589, 7885288, 7886571, 10299905, 11109211, 14780888, 14830102, 36888902, 46391557, 46391831, 57280005, 57404747, 75458863, 81063758, 99444027, 103165103, 103961278, 104636574, 127561235, 134340236, 134347923, 137098396, 142466817, 160649712, 160968629, 170481306, 196409719, 224991313, 229481470
|
|||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | Synthesis and biological evaluation of biphenylsulfonamide carboxylate aggrecanase-1 inhibitors. Bioorg Med Chem Lett. 2006 Jan 15;16(2):311-6. | |||
| REF 2 | The discovery of a potent and selective lethal factor inhibitor for adjunct therapy of anthrax infection. Bioorg Med Chem Lett. 2006 Feb 15;16(4):964-8. | |||
| REF 3 | Carbonic anhydrase and matrix metalloproteinase inhibitors. Inhibition of human tumor-associated isozymes IX and cytosolic isozyme I and II with su... Bioorg Med Chem. 2007 Mar 15;15(6):2298-311. | |||
| REF 4 | Heterocycle-based MMP inhibitors with P2' substituents. Bioorg Med Chem Lett. 2001 Apr 23;11(8):1009-13. | |||
| REF 5 | Methotrexate gamma-hydroxamate derivatives as potential dual target antitumor drugs. Bioorg Med Chem. 2007 Feb 1;15(3):1266-74. | |||
| REF 6 | Picking the S1, S1' and S2' pockets of matrix metalloproteinases. A niche for potent acyclic sulfonamide inhibitors. Bioorg Med Chem Lett. 1999 Jun 21;9(12):1691-6. | |||
| REF 7 | Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002 Sep;2(9):657-72. | |||
| REF 8 | Design, synthesis, and biological evaluation of potent thiazine- and thiazepine-based matrix metalloproteinase inhibitors. J Med Chem. 1999 Nov 4;42(22):4547-62. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

