Drug Information
| Drug General Information | Top | |||
|---|---|---|---|---|
| Drug ID |
D02DMQ
|
|||
| Former ID |
DAP000008
|
|||
| Drug Name |
Eletriptan
|
|||
| Synonyms |
Eletriptanum; Relpax; UK 116044; UK116044; Eletriptan (INN); Eletriptan [INN:BAN]; Relpax (TN); UK-116044; UK-116044-04; (R)-3-[(1-Methyl-2-pyrrolidinyl)methyl]-5-[2-(phenylsulfonyl)ethyl]-1H-Indole; (R)-5-[2-(Benzenesulfonyl)ethyl]-3-[(N-methylpyrrolidin-2-yl)methyl]-1H-indole; 3-(((R)-1-Methyl-2-pyrrolidinyl)methyl)-5-(2-(phenylsulfonyl)ethyl)indole; 3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-5-[2-(phenylsulfonyl)ethyl]-1H-indole; 5-[2-(benzenesulfonyl)ethyl]-3-[[(2R)-1-methylpyrrolidin-2-yl]methyl]-1H-indole
Click to Show/Hide
|
|||
| Drug Type |
Small molecular drug
|
|||
| Indication | Migraine [ICD-11: 8A80; ICD-10: G43, G43.9; ICD-9: 346] | Approved | [1], [2] | |
| Therapeutic Class |
Antimigraine Agents
|
|||
| Company |
Pfizer Inc
|
|||
| Structure |
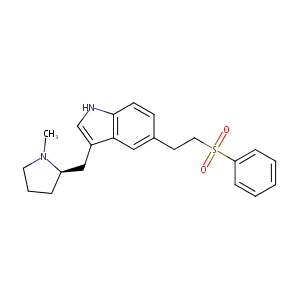 |
Download2D MOL |
||
| Formula |
C22H26N2O2S
|
|||
| Canonical SMILES |
CN1CCCC1CC2=CNC3=C2C=C(C=C3)CCS(=O)(=O)C4=CC=CC=C4
|
|||
| InChI |
1S/C22H26N2O2S/c1-24-12-5-6-19(24)15-18-16-23-22-10-9-17(14-21(18)22)11-13-27(25,26)20-7-3-2-4-8-20/h2-4,7-10,14,16,19,23H,5-6,11-13,15H2,1H3/t19-/m1/s1
|
|||
| InChIKey |
PWVXXGRKLHYWKM-LJQANCHMSA-N
|
|||
| CAS Number |
CAS 143322-58-1
|
|||
| PubChem Compound ID | ||||
| PubChem Substance ID |
8199306, 12014973, 14829466, 14927226, 43133578, 46506380, 50070538, 50071173, 50125841, 50462554, 53790006, 56394955, 92720041, 96024583, 103558413, 104368729, 109693042, 121264533, 126620364, 126652688, 126667007, 128979846, 134337338, 135035470, 135650227, 137002442, 142037588, 144206290, 151990438, 160963564, 163134331, 164814960, 170465317, 175265777, 175437670, 179149922, 196108133, 198993164, 223554905, 223659237, 223824718, 226414505, 249804705, 251916767, 251918006, 252215339
|
|||
| ChEBI ID |
CHEBI:50922
|
|||
| ADReCS Drug ID | BADD_D00755 ; BADD_D00756 | |||
| SuperDrug ATC ID |
N02CC06
|
|||
| SuperDrug CAS ID |
cas=143322581
|
|||
| Target and Pathway | Top | |||
|---|---|---|---|---|
| Target(s) | 5-HT 1B receptor (HTR1B) | Target Info | Modulator | [3] |
| 5-HT 1D receptor (HTR1D) | Target Info | Modulator | [3] | |
| KEGG Pathway | cAMP signaling pathway | |||
| Neuroactive ligand-receptor interaction | ||||
| Serotonergic synapse | ||||
| Panther Pathway | Heterotrimeric G-protein signaling pathway-Gi alpha and Gs alpha mediated pathway | |||
| 5HT1 type receptor mediated signaling pathway | ||||
| Reactome | Serotonin receptors | |||
| G alpha (i) signalling events | ||||
| WikiPathways | Serotonin HTR1 Group and FOS Pathway | |||
| Monoamine GPCRs | ||||
| GPCRs, Class A Rhodopsin-like | ||||
| GPCR ligand binding | ||||
| GPCR downstream signaling | ||||
| References | Top | |||
|---|---|---|---|---|
| REF 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 40). | |||
| REF 2 | Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77. | |||
| REF 3 | Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. | |||
If You Find Any Error in Data or Bug in Web Service, Please Kindly Report It to Dr. Zhou and Dr. Zhang.

